Roxithromycin and ambroxol hydrochloride dispersible tablets
A technology of roxithromycin and ambroxol, applied in the direction of organic active ingredients, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of high cost, drug safety and bioavailability Influence, high excipients and other issues, to achieve the effect of convenient taking, stable inspection indicators, and small tablet weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1: preparation example
[0018] Grind ambroxol hydrochloride and roxithromycin respectively, pass through a 100-mesh sieve, take roxithromycin 150g, ambroxol hydrochloride 30g, mix with microcrystalline cellulose 50g, crospovidone 30g, add 1% Soft material made from 80ml of hypromellose 50% ethanol solution, passed through a 16-mesh sieve, dried at 50°C for two hours, stirred from time to time, granulated with a 18-mesh sieve, added with 3g of magnesium stearate and 10g of talcum powder, mixed evenly, and measured Particle content, compressed into tablets, ready to be obtained.
Embodiment 2
[0019] Embodiment 2: comparative test result
[0020] The inventor made a batch of samples (batch number: 071001) according to the method disclosed in the invention patent of application number 200610007513.0, and carried out content, related substances, dissolution rate and stability with the sample of the present invention (method of embodiment 1, batch number: 071002). (accelerated, long-term) comparative study, the results are as follows.
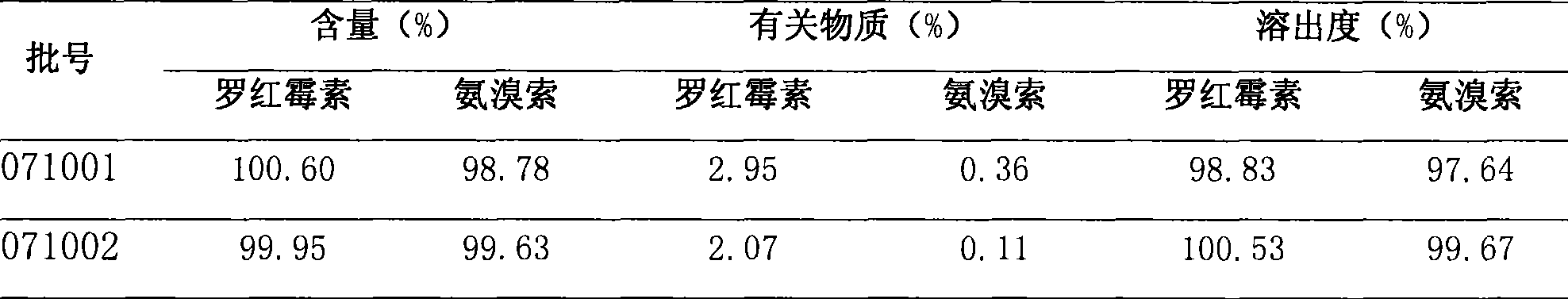
[0021] 1. Comparison of content, related substances and dissolution rate: See Table 2 for the results.
[0022] Table 2 Content, related substances, dissolution comparison test results
[0023]
[0024] The test results showed that the related substances of the 071002 batch of samples were significantly less than those of the 071001 batch, and there was no significant difference in content and dissolution rate.
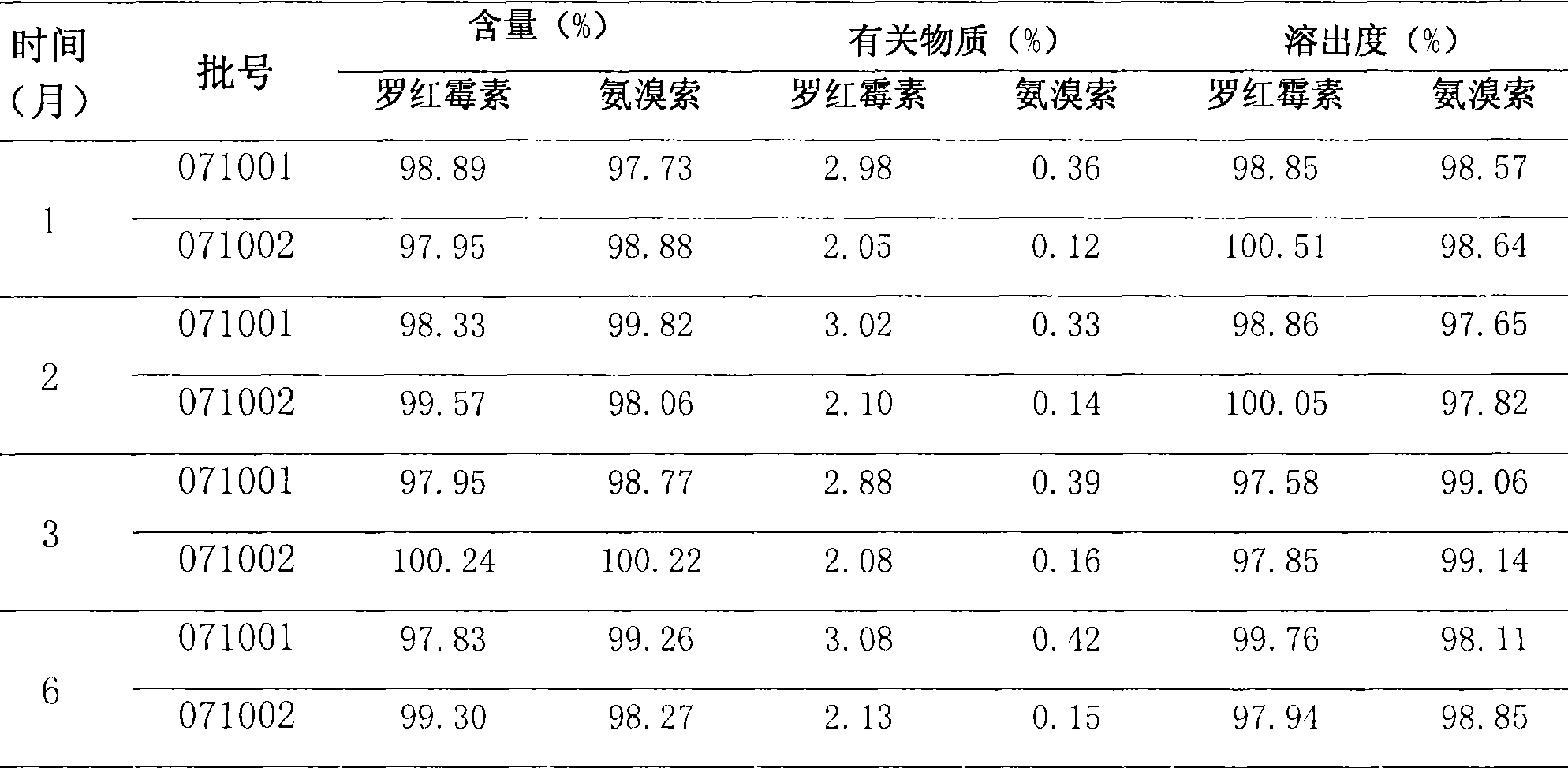
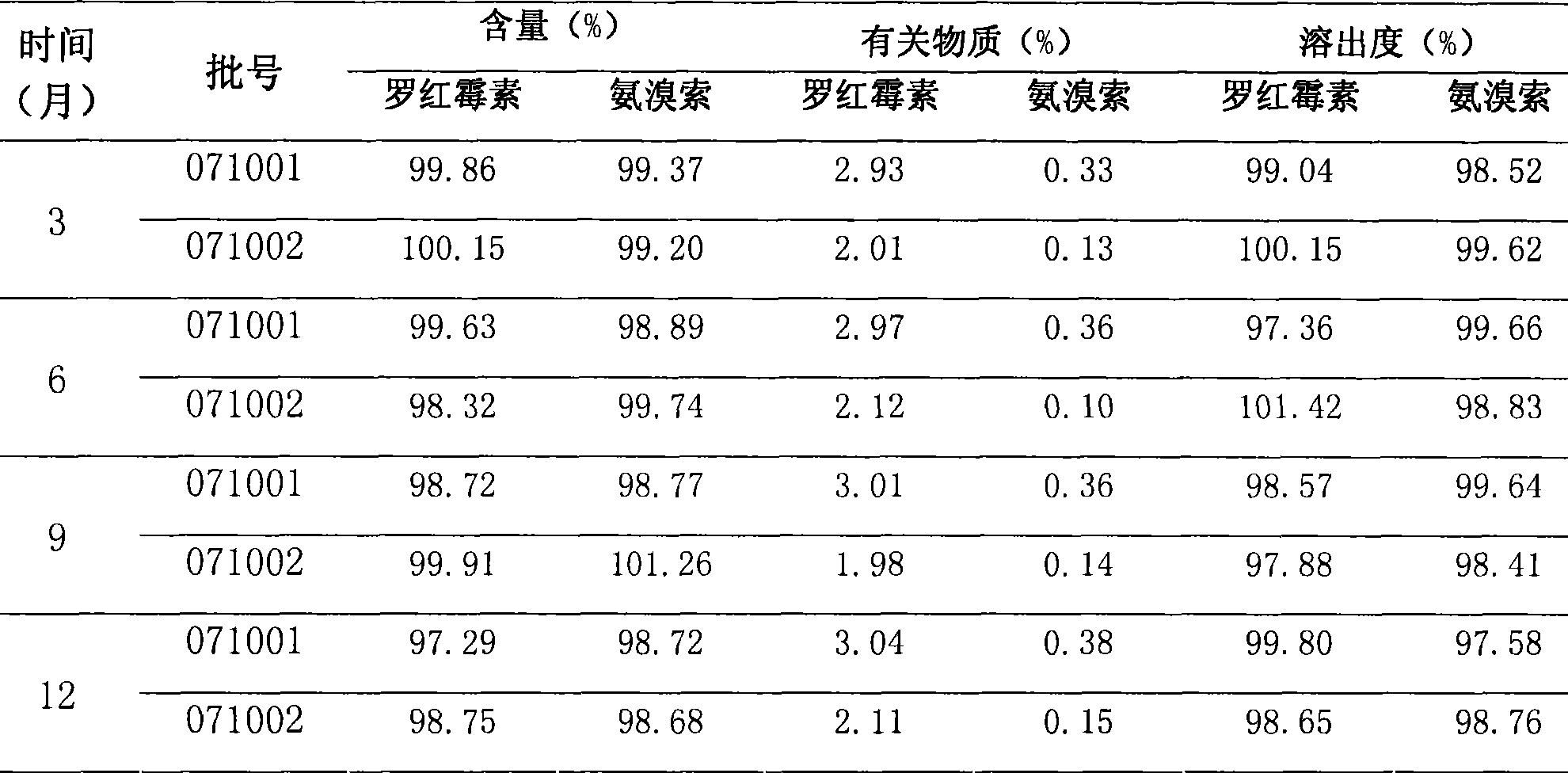
[0025] 2. Stability test comparison: the results are shown in Tables 3 and 4.
[0026] Table 3 Accelerated test (40°C, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com