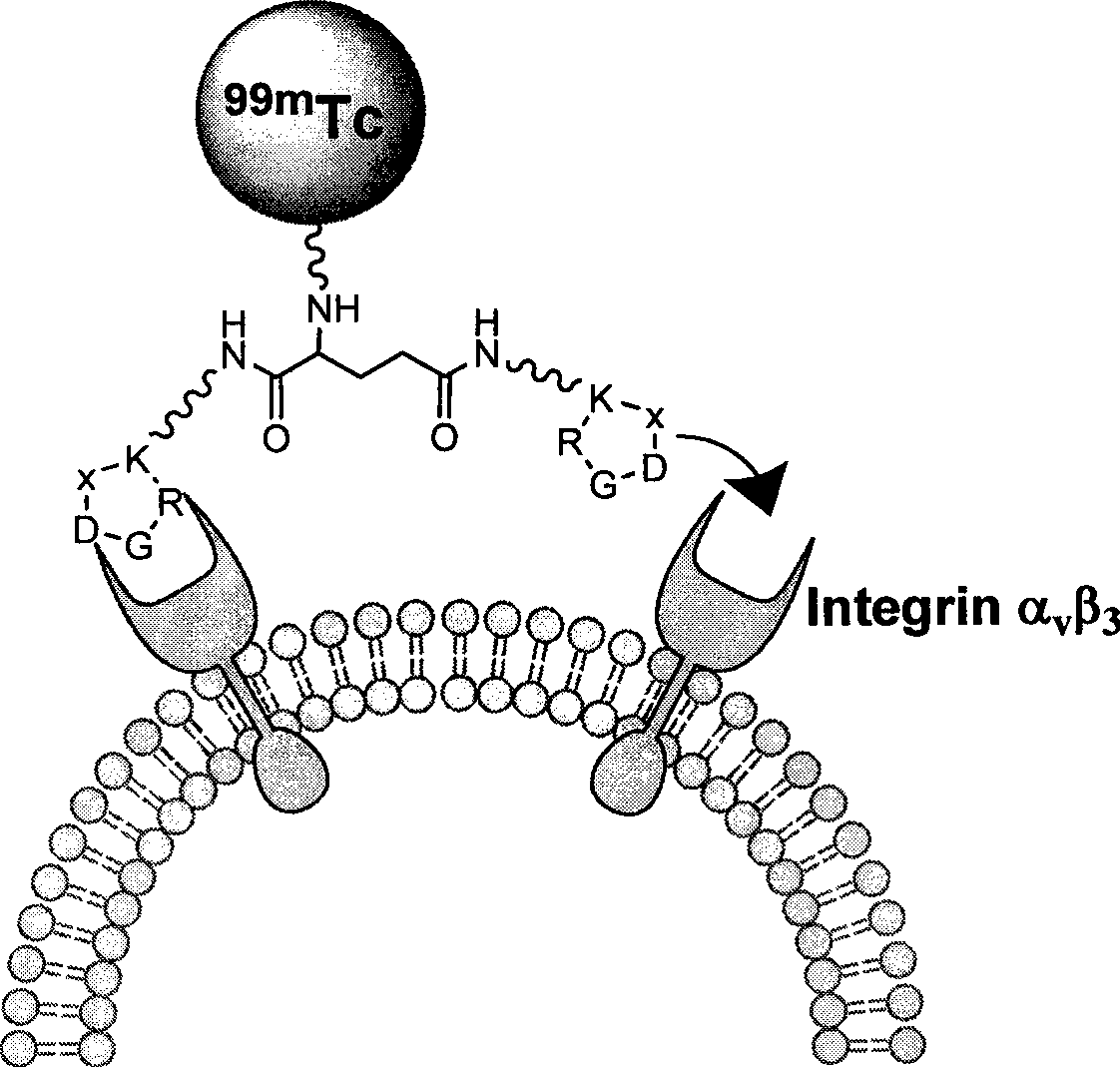
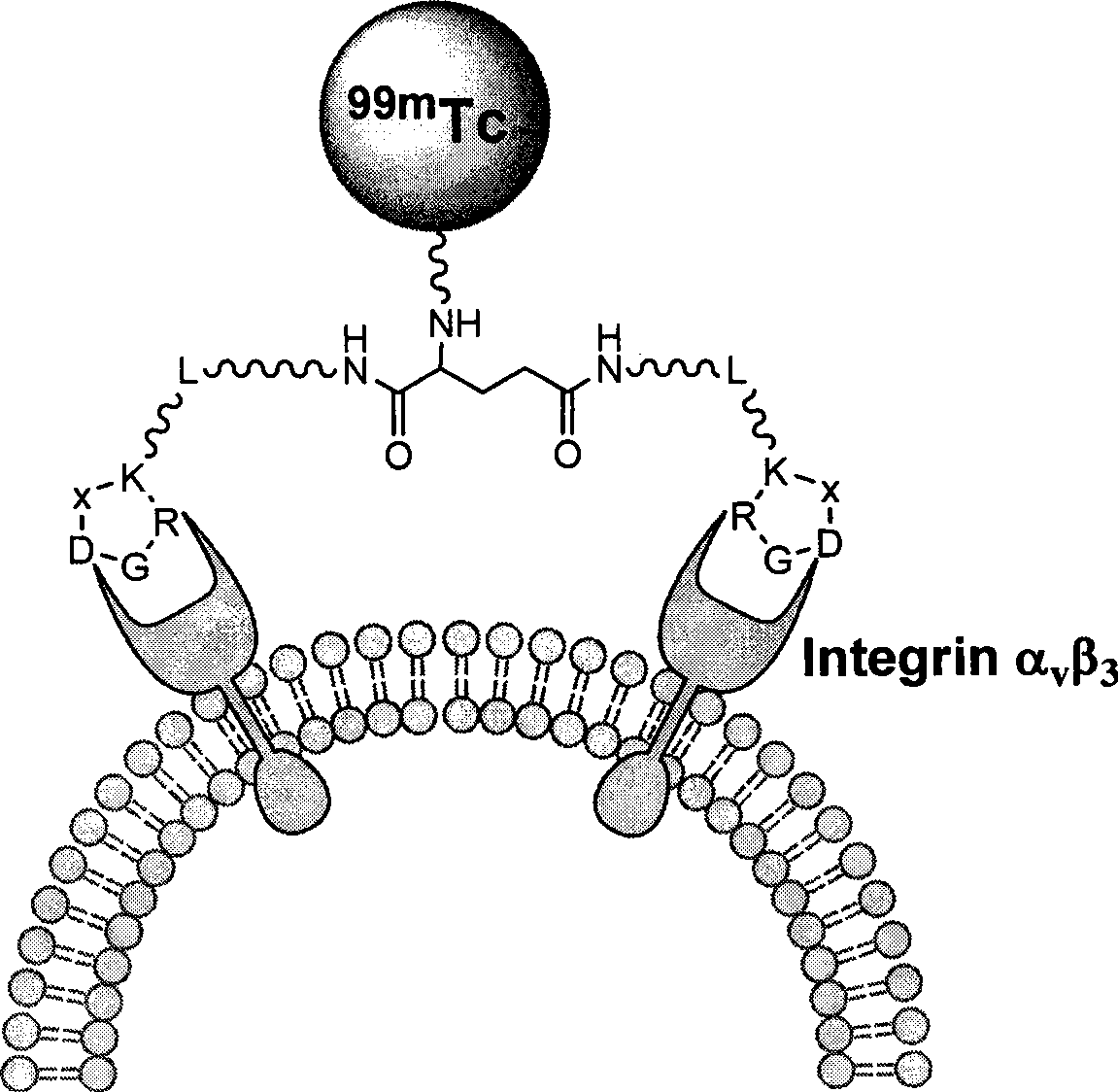
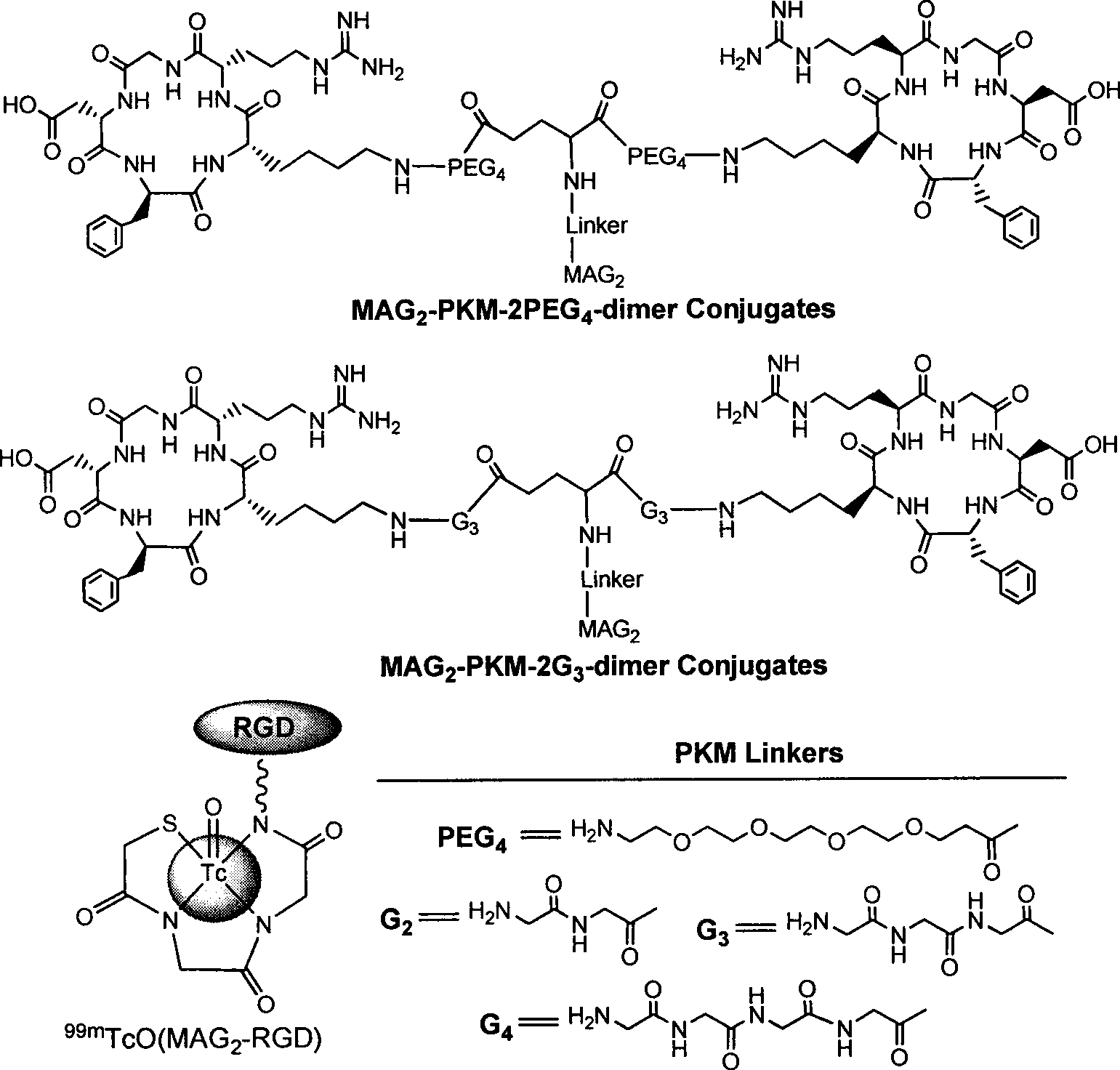
Radioactive nuclide marked RGD polypeptide medicament and preparation method thereof
A radionuclide and labeling technology, applied in the field of radionuclide-labeled RGD polypeptide drugs and their preparation, can solve problems such as insufficient distance, achieve good diagnosis and treatment effects, enhance binding affinity, and improve pharmacokinetic properties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] In this example, the radionuclide 99m Tc-labeled RGD peptide drug 99m Tc-MAG 2 -PEG 4 -E[PEG 4 -c(RGDfK)] 2 (referred to as 99m Tc-MAG 2 -3PEG 4 -dimer) and its preparation method as an example.
[0034] exist 99m Tc-MAG 2 -PEG 4 -E[PEG 4 -c(RGDfK)] 2 Among them, the RGD cyclic peptide dimer is the linker PEG 4 Linked with RGD polypeptide monomer c (RGDfK), and then two linked with PEG 4 The RGD cyclic peptide dimer synthesized by the dimerization of the RGD polypeptide monomer, that is, E[PEG 4 -c(RGDfK)] 2 , radionuclide 99m Tc through a bifunctional chelator MAG 2 labeling the RGD cyclic peptide dimer, and a pharmacokinetic modification molecule PEG is also connected between the RGD cyclic peptide dimer and the bifunctional chelating agent 4 , the radionuclide-labeled RGD polypeptide drug is a colorless transparent liquid injection.
[0035] The drug first combines four molecules of polyethylene glycol (PEG 4 , PEG=Polyethylene glycol) is connecte...
Embodiment 2
[0055] In this example, the radionuclide 188 Re-labeled RGD peptide drug 188 Re-MAG 2 -PEG 4 -E[PEG 4 -c(RGDfK)] 2 (referred to as 188 Re-MAG 2 -3PEG 4 -dimer) as an example.
[0056] exist 188 Re-MAG 2 -PEG 4 -E[PEG 4 -c(RGDfK)] 2 Among them, the RGD cyclic peptide dimer is the linker PEG 4 Linked with RGD polypeptide monomer c (RGDfK), and then two linked with PEG 4 The RGD cyclic peptide dimer synthesized by the dimerization of the RGD polypeptide monomer, that is, E[PEG 4 -c(RGDfK)] 2 , radionuclide 188 Re through a bifunctional chelating agent MAG 2 labeling the RGD cyclic peptide dimer, and a pharmacokinetic modification molecule PEG is also connected between the RGD cyclic peptide dimer and the bifunctional chelating agent 4 , the radionuclide-labeled RGD polypeptide drug is a colorless transparent liquid injection.
[0057] The radionuclide-labeled RGD polypeptide drug of the present embodiment is 188 Re-MAG 2 -PEG 4 -E[PEG 4 -c(RGDfK)]2 , the dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com