Pharmaceutical composition for treating or preventing hcv infection
A composition and drug technology, applied in the direction of drug combination, antineoplastic drugs, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
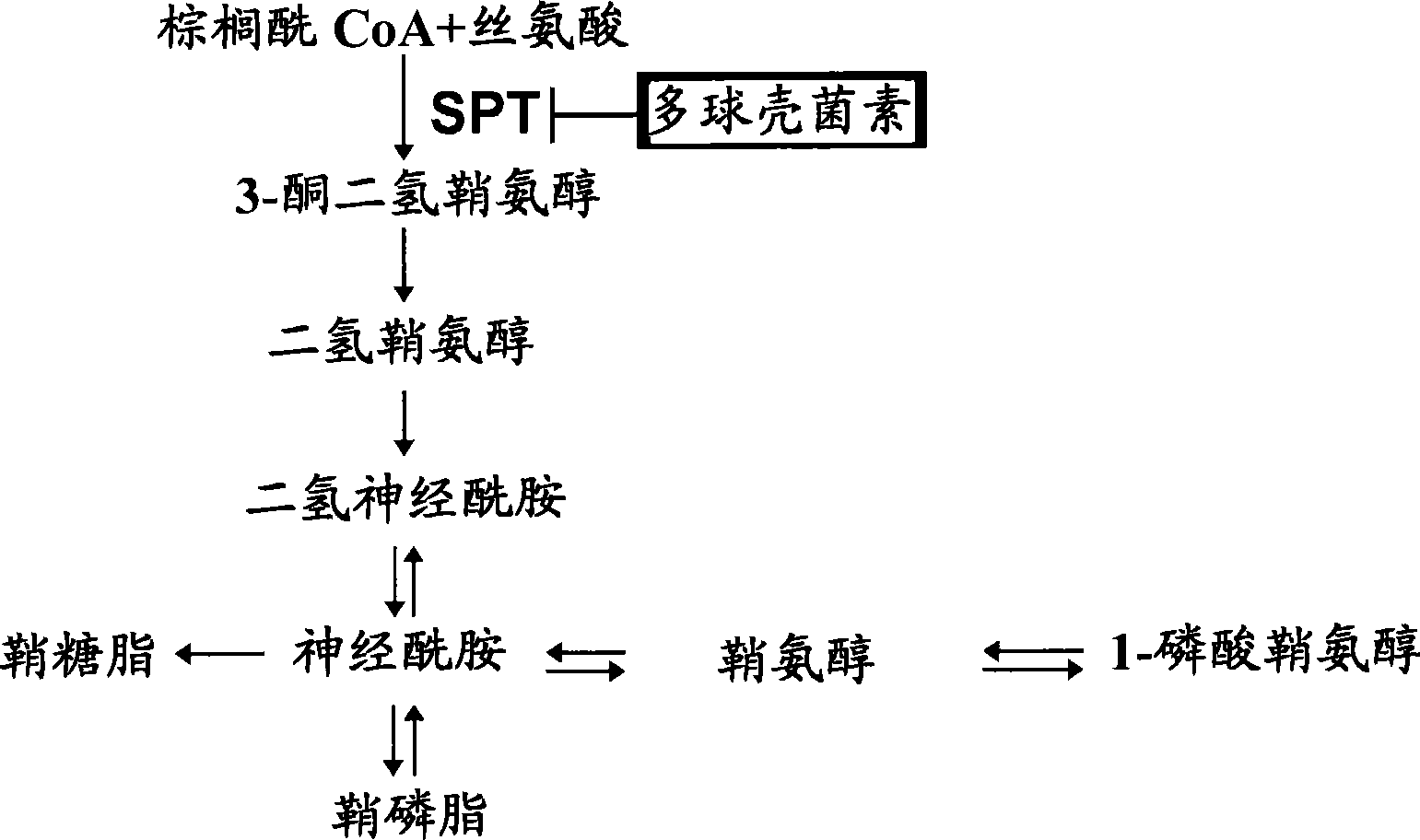
[0296] [Example 1] Anti-HCV effect of SPT inhibitor myriocin
[0297] The present inventors studied the anti-HCV effect and cytotoxicity of myriocin in the HCV subgenome replicon cell FLR3-1.
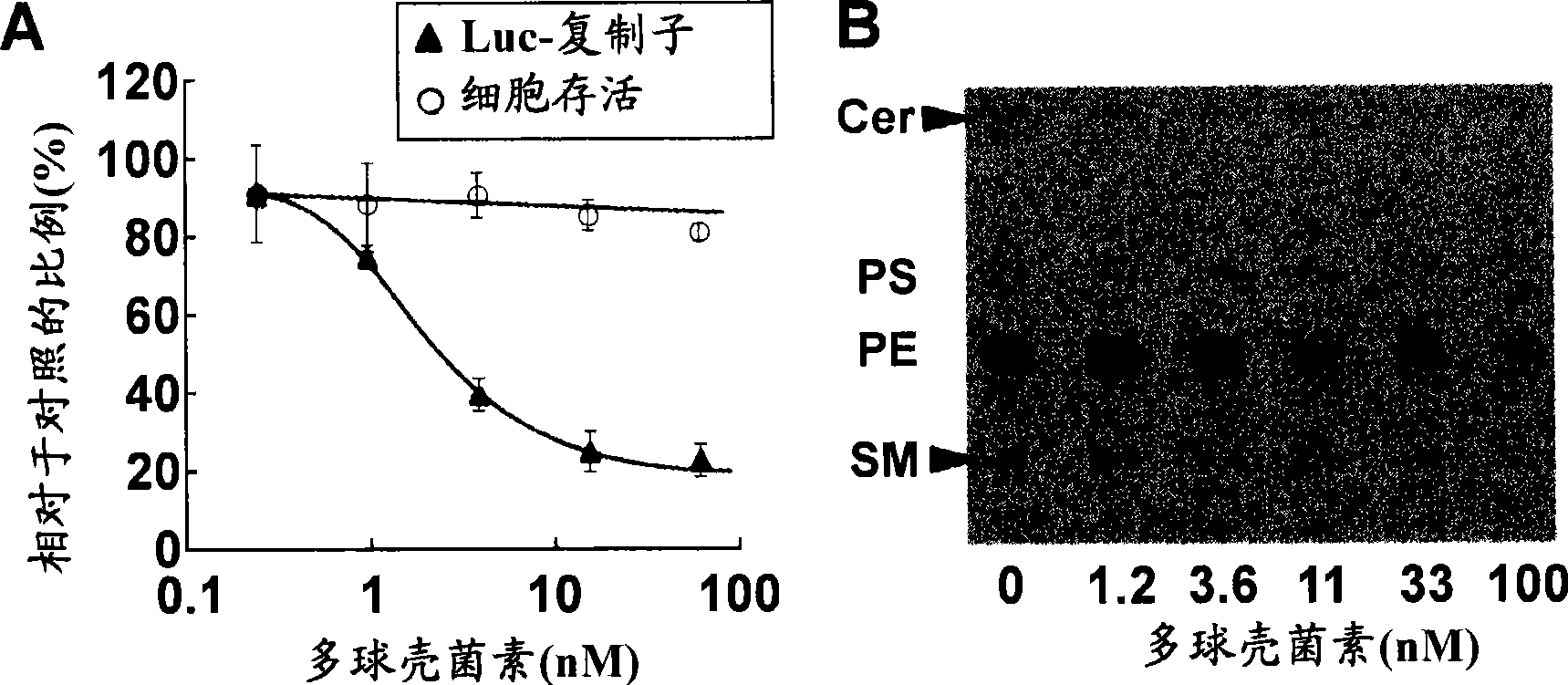
[0298]First, myriocin (Sigma, St. Louis, MO, USA) was added to HCV subgenomic replicon cells FLR3-1 (genotype 1b, Con-1, Sakamoto, H. et al., Nat Chem Biol, 1, 333-337 (2005)) in the proliferation medium, the final concentration was 0.2, 1.0, 3.9, 15.6 or 62.5 nM. After culturing for 72 hours, the inventors performed a luciferase assay using the Birght-Glo Luciferase Assay Kit (Promega, Madison, Wisconsin, USA).
[0299] In addition, myriocin was added to FLR3-1 cells, cultured for 72 hours, and then the cell viability was measured using Tetra Color One kit (Seikagakukogyo, Tokyo, Japan) according to the manufacturer's instructions.
[0300] The results showed that myriocin had no effect on cell viability ( figure 2 A) or cell proliferation (data not shown) is affected, but the lu...
Embodiment 2
[0304] [Example 2] Correlation between sphingolipid metabolites and HCV replication
[0305] To investigate the correlation of sphingolipid metabolites with HCV replication, the present inventors monitored de novo biosynthesis of sphingolipids in FLR3-1 cells in the presence of myriocins.
[0306] First, FLR3-1 cells were mixed in Opti-MEM (Invitrogen) with [ 14 C] Serine (0.5 μCi / mL) was incubated together for 2 hours. Cells were lysed with 0.1% SDS, and then total lipids were extracted with chloroform / methanol (1:2 v / v). The extract was spotted on a silica gel 60 thin-layer chromatography (TLC) plate (Merck, Darmstadt, Germany) by methyl acetate / 1-propanol / chloroform / methanol / 0.25% KCl (25:25: 25:10:9, v / v) for chromatographic analysis. Radioactive spots were detected by BAS 2000 (Fuji Film, Kanagawa, Japan).
[0307] As a result, the production of ceramide and sphingomyelin was dose-dependently inhibited, while the production of phosphatidylethanolamine and phosphatid...
Embodiment 3
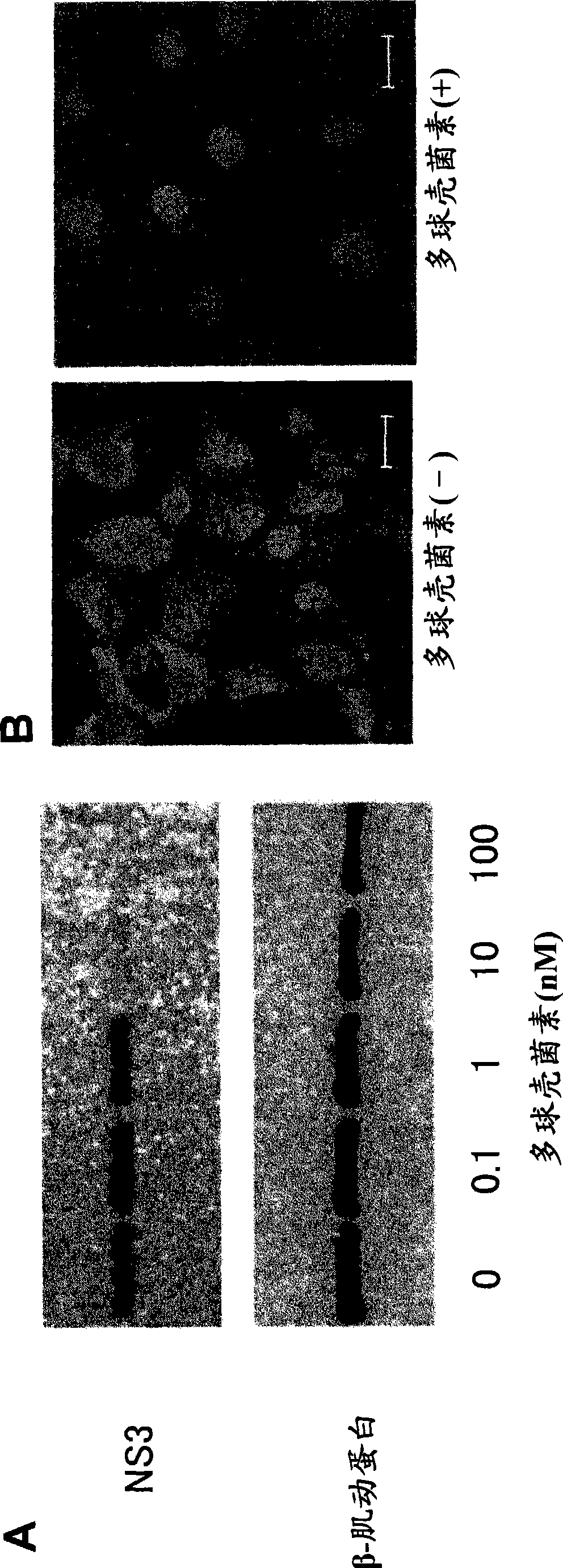
[0311] [Example 3] Infection of HCV in chimeric mice against myriocin and PEG-IFN HCV effect
[0312] The inhibitory capacity of myriocins was studied using chimeric mice with humanized livers infected with HCR6 (genotype 1b). The chimeric mice used were purchased (PhoenixBio Co., Ltd., Hiroshima, Japan).
[0313] Specifically, according to Table 2, myriocin and / or PEG-IFN (Chugai, Tokyo, Japan) were administered to patients infected with HCV genotype 1b (HCR6, accession number) by intraperitoneal or subcutaneous injection. AY045702) mice, blood was collected.
[0314] (Table 2) Dosing schedule for chimeric mice infected with HCV genotype 1b
[0315]
[0316] In Table 2, B, I, and M show that each operation was performed as needed, and that the administration of the reagent was started from day 0. PEG-IFN was injected at 30 μg / kg. The amount of myriocin injected was adjusted according to the body weight of the mice. Dosing starts at 1 mg / kg (M), and when the body w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com