Multi-shell rhzomorph derivative, preparation and uses thereof
A technology of myriocin and its derivatives, which is applied to measurement devices, instruments, scientific instruments, etc., can solve the problems of low content of myriocin, low sensitivity, complicated operation, etc., and achieve the effect of reliable measurement results.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation of embodiment 1 formula (I) compound
[0030] Preparation of fluorenylmethoxycarbonyl chloride (Fmoc-Cl) solution: Accurately weigh about 5.0 mg of Fmoc-Cl, dissolve it in 50 mL of tetrahydrofuran solution, and prepare a derivatizer solution containing 0.1 mg of Fmoc-Cl per 1 mL, and store in a refrigerator at 4°C spare.
[0031] Preparation of myriocin solution: Accurately weigh about 2.5mg of myriocin, put it in a 50mL measuring bottle, dissolve it with pyridine and dilute to the mark, shake well, and make 0.05mg of myriocin per 1mL The solution was stored in a 4°C refrigerator for later use.
[0032] Measure 100.0 μl of the above-mentioned Fmoc-Cl solution and 50.0 μl of the myriocin solution respectively, put them in a 1.5mL Eppendorf tube, mix well, and the reaction temperature is 40° C., and the reaction time is 10 minutes to obtain the reactant containing the compound of formula (I). Dry the solvent in the reaction product, and the residue is pu...
Embodiment 2
[0033] The preparation of embodiment 2 formula (I) compound
[0034] Preparation of fluorenylmethoxycarbonyl chloride (Fmoc-Cl) solution: Accurately weigh about 20.0 mg of Fmoc-Cl, dissolve it in 100 mL of tetrahydrofuran solution, and prepare a derivative solution containing 0.2 mg of Fmoc-Cl per 1 mL, and store in a refrigerator at 4°C spare.
[0035] Preparation of myriocin solution: Accurately weigh about 2.0 mg of myriocin, put it in a 10 mL measuring bottle, dissolve it with pyridine and dilute to the mark, shake well, and make 0.2 mg of myriocin per 1 mL The solution was stored in a 4°C refrigerator for later use.
[0036] Measure 100.0ml of the above-mentioned Fmoc-Cl solution and 10.0ml of the myriocin solution respectively, put them in a 500mL round-bottomed flask, and mix them well. The reaction temperature is 50°C, and the reaction time is 20min to obtain the reactant containing the compound of formula (I). Dry the solvent in the reaction product, and the residue...
Embodiment 3
[0037] Embodiment 3 High performance liquid chromatography-mass spectrometry qualitative analysis formula (I) compound
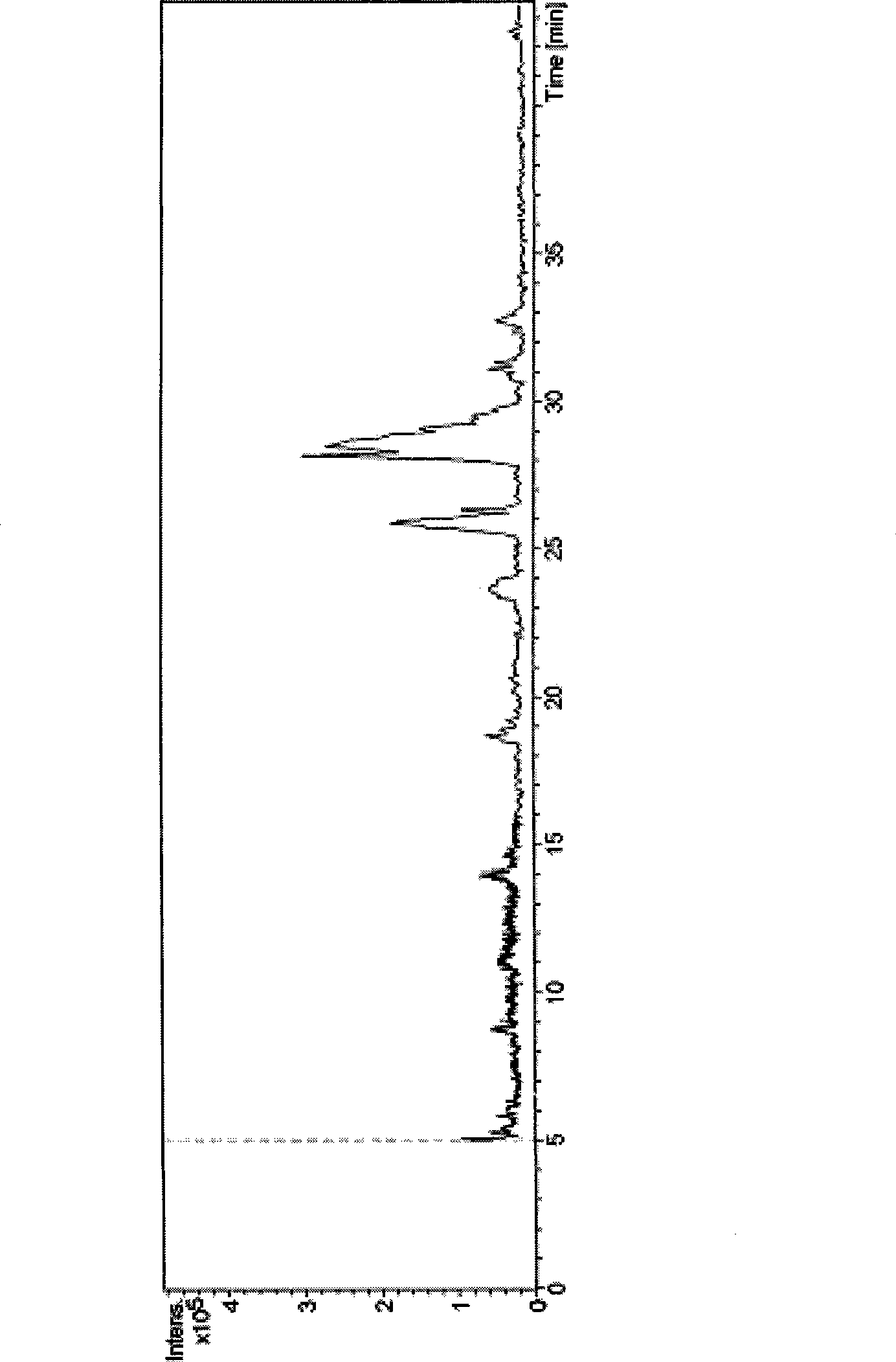
[0038] 10 μL of the reactant prepared in Example 1 was injected into a high-performance liquid chromatography-electrospray ionization-mass spectrometer (HPLC-ESI-MS) for analysis. Chromatographic column: C8 chromatographic column; mobile phase: volume ratio of acetonitrile to water 70:30; flow rate 1.0mL / min; column temperature 35°C, detection by mass spectrometer.
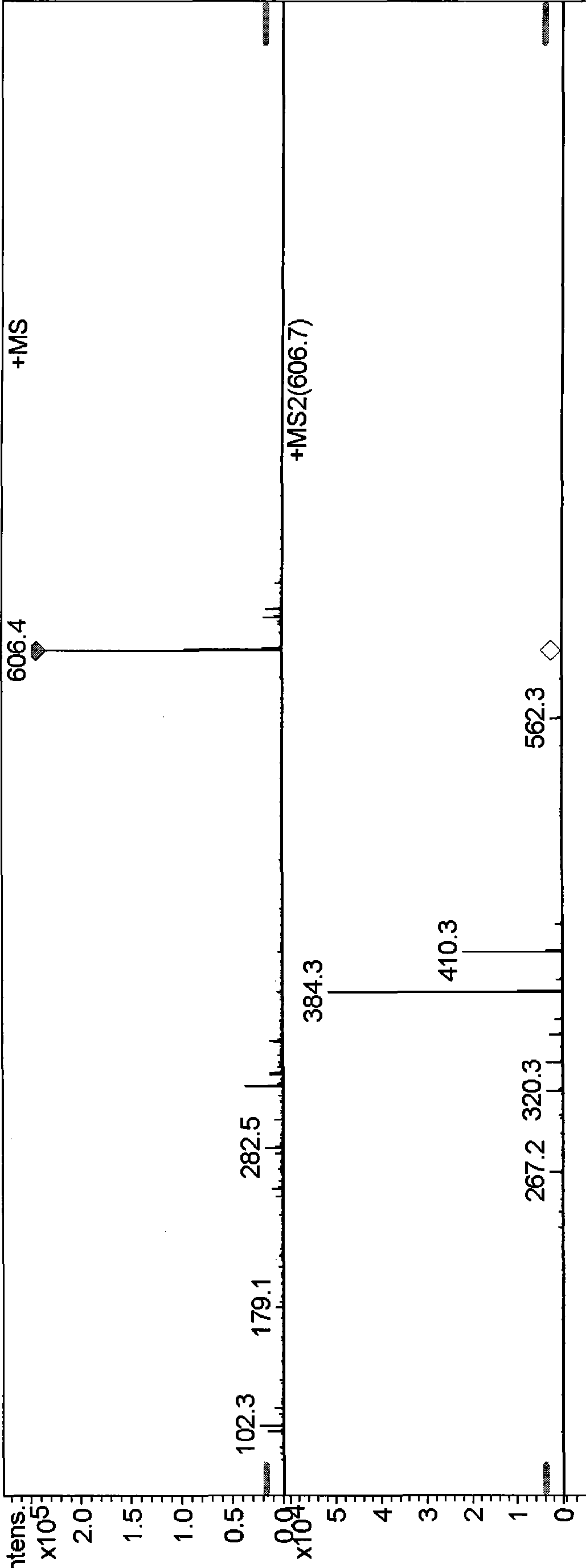
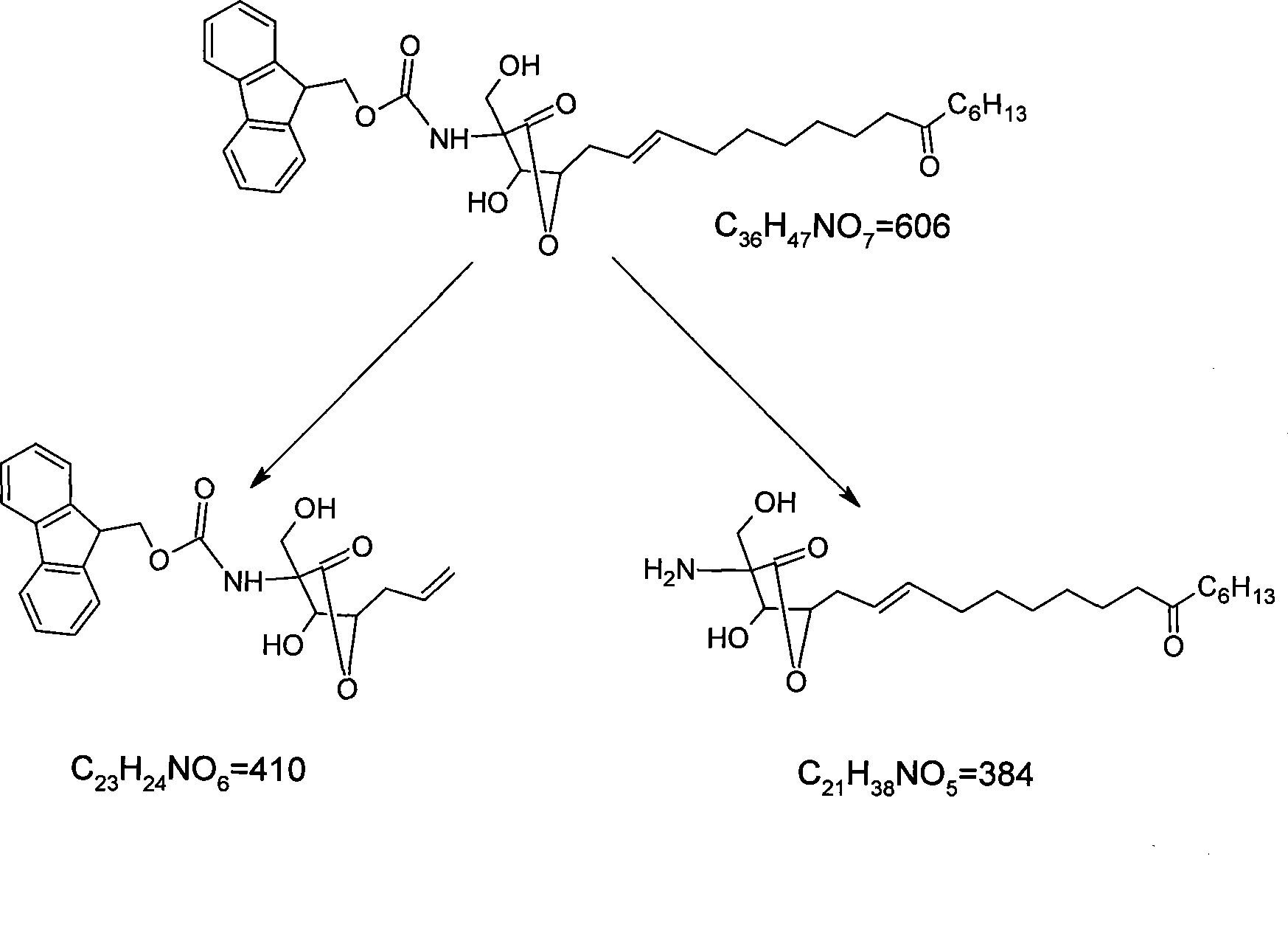
[0039] In ESI positive ion full scan mode, [M+H] can be obtained + Be the quasi-molecular ion peak of 606.4, the molecular weight of formula (I) compound is 605, speculate [M+H] + The quasi-molecular ion peak of 606.4 is the quasi-molecular ion peak of the compound of formula (I). Select m / z606.4 to carry out secondary mass spectrometry analysis, obtain its secondary mass spectrum, can observe that m / z is the fragmentation peak of 384.3 and 410.3, and the m / z that can also be cracked under the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com