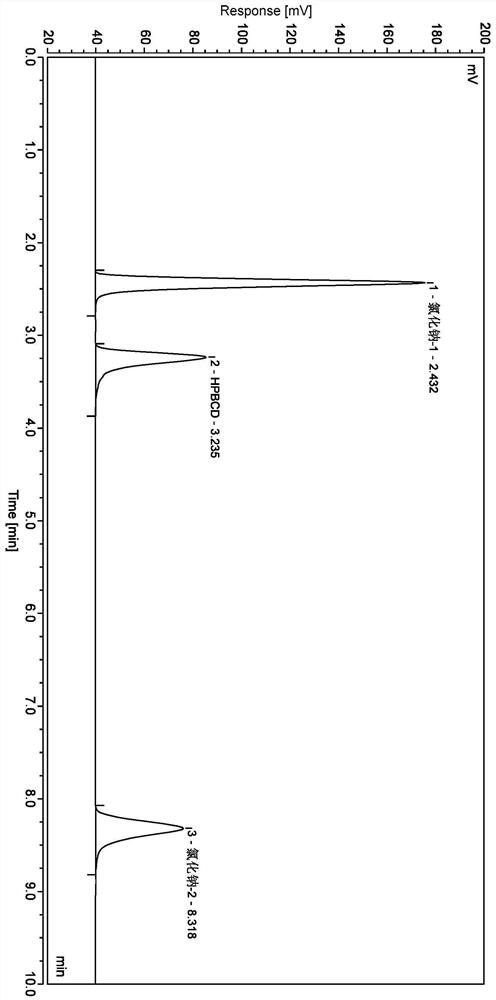
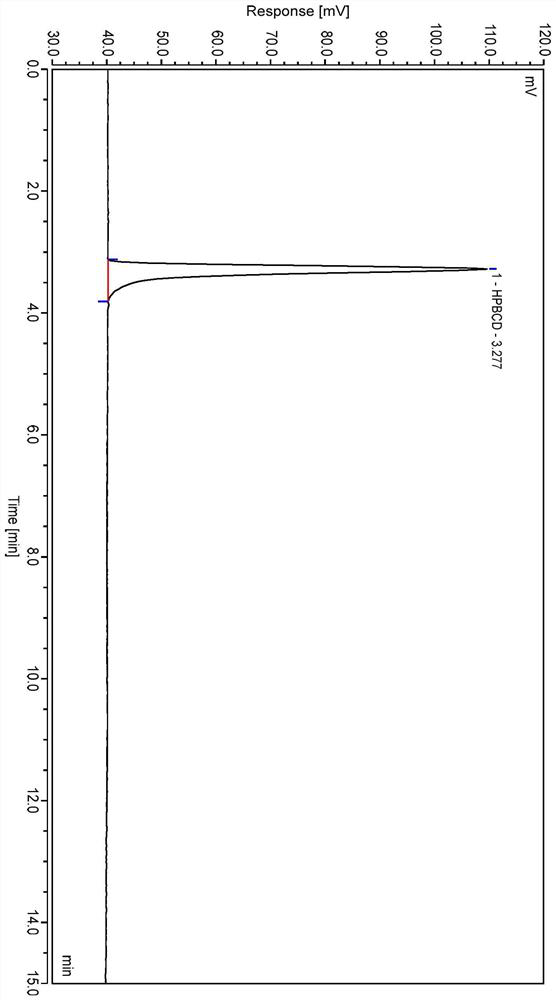
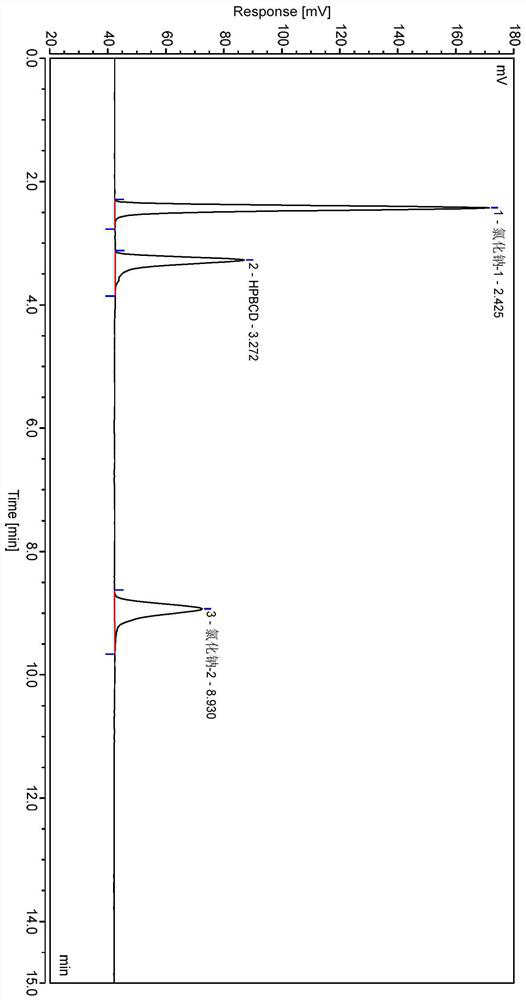
Method for analyzing content of hydroxypropyl-beta-cyclodextrin in butylphthalide sodium chloride injection
A technology of beta-cyclodextrin and analysis method, which is applied in the field of analysis of hydroxypropyl beta-cyclodextrin content in butylphthalide injection, and can solve the complicated operation process and the peak shape of hydroxypropyl beta-cyclodextrin. problems such as poor analysis methods and long time consumption of analysis methods, to achieve the effect of accurate measurement results and reliable quality control methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] High performance liquid chromatography conditions:
[0032] The chromatographic column is a cyanosilane bonded silica gel column thermo hypersil GOLD CN (250 × 4.67 mm, 5 μm), and the flow phase A of 5 mmol / L acetate is a flow phase A, with acetonitrile to flow phase B, flow rate of 1.0 mL / min, column Temperate 20 ° C, the injection volume is 10 μL, evaporated light scatter detector (parameter: carrier gas pressure: 0.5MPa; drift tube temperature: 70 ° C; carrier gas flow rate: 2.0ml / min; diversion mode), press the table wash Take off.
[0033] Table 1 elution program
[0034]
[0035] Sample formulation:
[0036] Blank excipient solution: Precision quantity taking 1ml of sodium phthalonate injection without hydroxypropylene cyclodextrin, placed in a 25 mL volumetric flask, diluted with water to the scale, shake well, to a blank excipient solution.
[0037] Systematic Solution: Take hydroxypropyloid cyclodextrin control and sodium chloride control, add water to diss...
Embodiment 2
[0043] High performance liquid chromatography conditions: in Example 1.
[0044] Sample formulation:
[0045] Systematic Solution: That Example 1.
[0046] Take the solution: Take the amount of hydroxypropyloid cyclodextrin, precisely, add water to dissolve and quantitatively dilute the solution of about 0.28 mg of hydroxypropylene rhythmic cyclodextrin per 1 ml, as a solution solution .
[0047] For test solution: in Example 1.
[0048] Determination: Applicable solution, refollective solution, and test solution solution, 10 μl of injection liquid chromatography in each precision amount after formulation of 0, 1, 2, 4, 6, 8, 12, 16, 20, 24 hours, respectively Record the chromatogram, calculate the results of 24h stability, and the results are shown in Table 2, Table 3, Table 4.
[0049] Table 2 Systematic Solution Stability Test Results
[0050]
[0051] Table 3 Test results for the stability test
[0052]
[0053] Table 4 Test results of solution solution solution
[0054]
...
Embodiment 3
[0057] High performance liquid chromatography conditions: in Example 1.
[0058] Sample formulation:
[0059] Hydroxypropyliphyde radioplasm Take the preparation reserve: It is called the hydroxypropyl propyride, and it is precisely known, and the 50mL volumetric flask is added, and the appropriate amount of water is ultrasonically dissolved and diluted to the scale, shake well , As a hydroxypropyl propyride cyclodextrin to the illumination stock solution.
[0060] Hydroxypropylphamphetama cyclodextrin series control solution: precisely precise amounts of hydroxypropyl dipyethylene cyclodextrin to 1ml, 1.5 mL, 2 mL, 2.5ml, 3ml in 10mL volume bottle, plus water To obtain a series of linear solutions for hydroxypropyliphyde radioptride.
[0061] Determination: Precision quantity takes the systematic solution, for the test solution, and 10 μl of the series of injectable solutions, and record chromatograms.
[0062] Table 5 Hydroxypropylbahtha Cyclodextrin Linearity and Range Test Res...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com