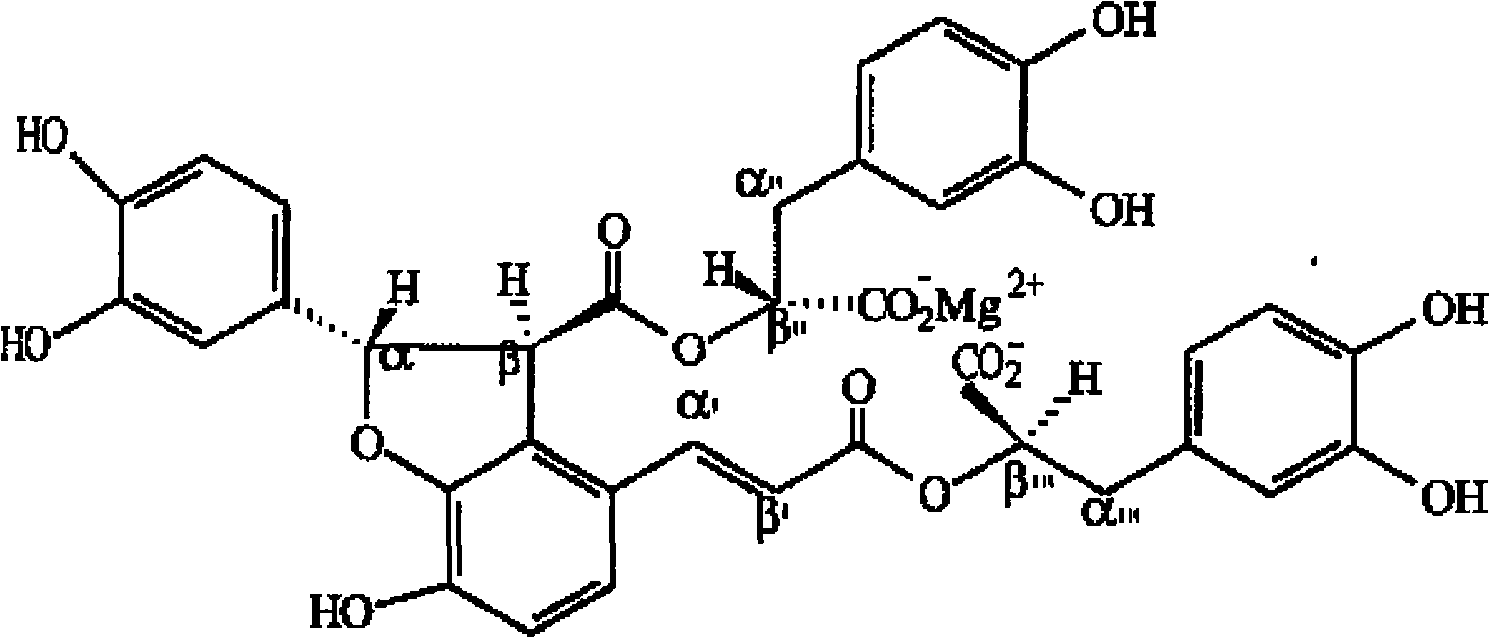
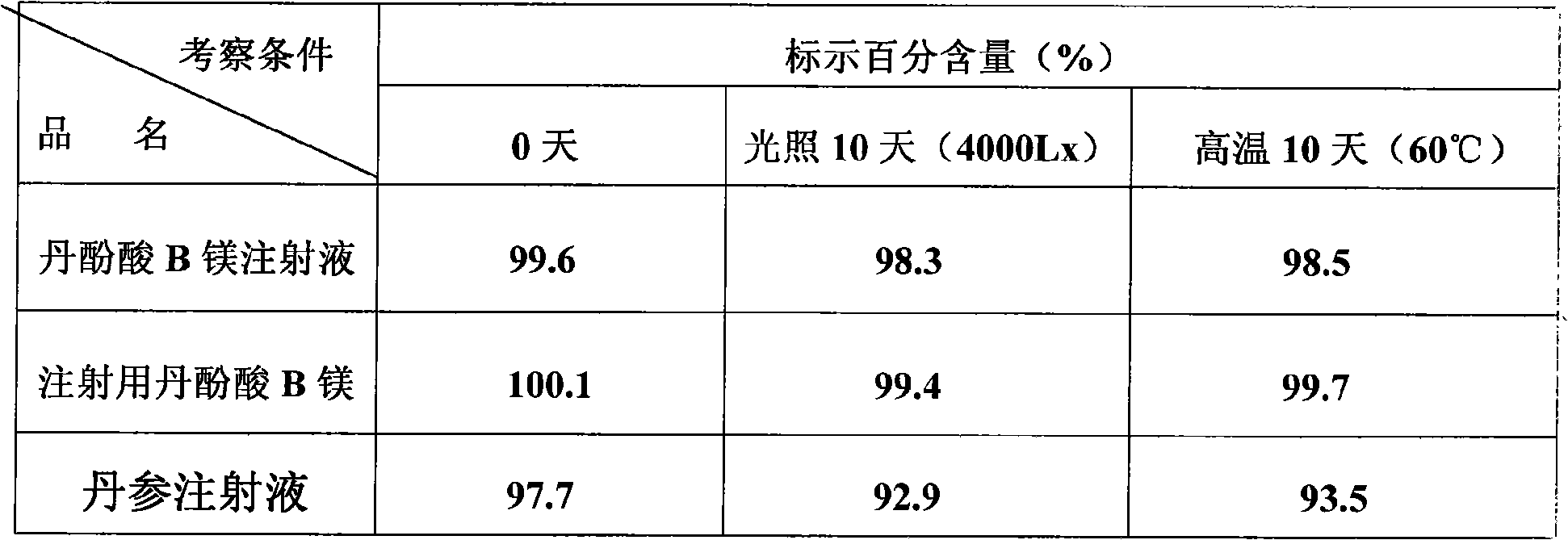
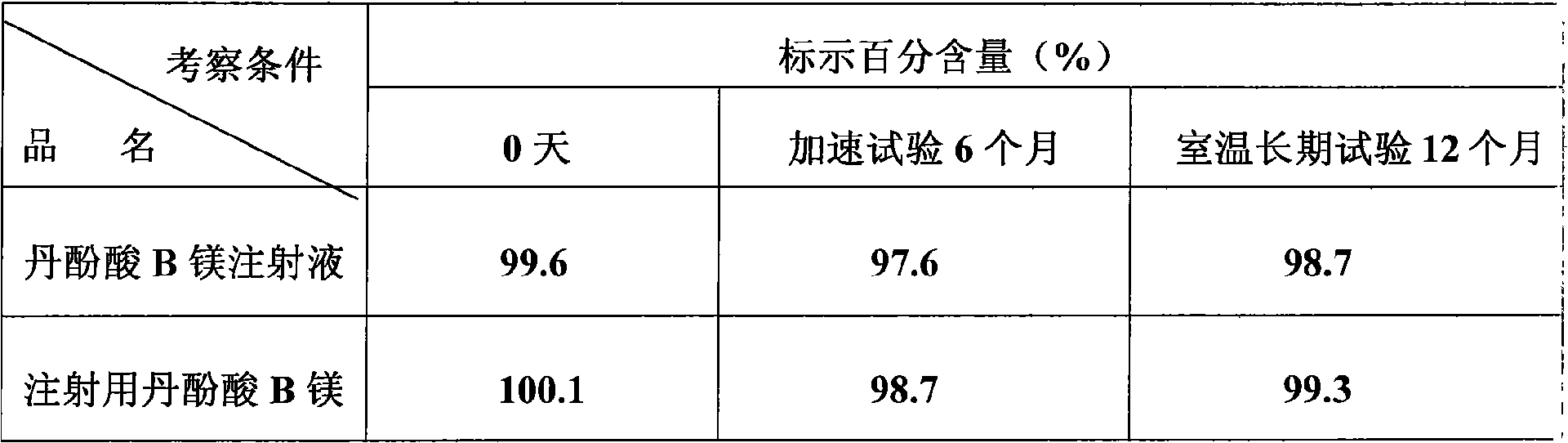
Salvianolic acid B magnesium injection, preparation method and use thereof
A technology of salvianolic acid and injection, which is applied in the field of injections for the treatment of cardiovascular and cerebrovascular diseases, and can solve problems such as light and heat sensitivity, unstable aqueous solution, and increased impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The preparation process of salvianolic acid B magnesium injection is as follows: take 0.1 g of sodium bisulfite and dissolve it in 50 ml of water for injection, add 80 mg of disodium edetate to dissolve and mix, and then dissolve 1 g of salvianolic acid B magnesium in Add appropriate amount of dilute hydrochloric acid solution to the above solution to adjust the pH to 3.0. Add 0.1% activated carbon for injection and stir at 50°C for 20 minutes, filter, add water for injection to the full volume of 100ml, and pass through a 0.22μm membrane to make 100 tubes, fill in ampoules, fill with nitrogen, and sterilize at 100°C for 30 minutes to obtain the finished product.
Embodiment 2
[0045] For the magnesium salvianolic acid B injection described in Example 1, take 0.1 g of sodium bisulfite and dissolve it in 100 ml of water for injection, add 80 mg of disodium edetate to dissolve and mix, then dissolve 1 g of magnesium salvianolic acid B Add an appropriate amount of acidic buffer salt to the above solution to adjust the pH to 3.5. Add 0.1% activated carbon for injection and stir at 50°C for 20 minutes, filter, add water for injection to 200ml, pass through a 0.22μm membrane, make 100 tubes, fill in ampoules, fill with nitrogen, and sterilize at 100°C for 30 minutes to obtain the finished product.
Embodiment 3
[0047]The magnesium salvianolic acid B injection described in embodiment 1, gets sodium bisulfite 0.5g, vitamin C 1.5g, disodium edetate 50mg is dissolved in 300ml water for injection, then the magnesium salvianolic acid B 5g Dissolve in the above solution, adjust the pH to 3.5 with an appropriate amount of dilute phosphoric acid, add 0.05% activated carbon for injection and stir at 50°C for 20 minutes, filter water for injection to a total volume of 500ml, and pass through a 0.22μm membrane to make 100 sticks. Fill in ampoules, fill with nitrogen, and sterilize at 100°C for 30 minutes to obtain the finished product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com