Complex of Fab fragment of C2H7 and CD20 antigen epitope polypeptide
An antigenic epitope, CD20 technology, applied in receptor/cell surface antigen/cell surface determinant, peptide preparation method, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc. Functional differences, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 , Antibody preparation
[0024]Refer to the method published by Liu, A.Y., Robinson, R.R. et al. on pages 3521-3526 of "Journal of Immunology" No. 139 to prepare antibodies, and digest C2H7 with papain. Add papain (Sigma-Aldrich) to a final concentration of 0.1mg / ml; then digest at 37°C for 18 hours, buffer: 0.1M Tris-HCl+2mM EDTA+1mM dithiothreitol, pH8.0 . The digested product was firstly subjected to cation exchange chromatography through a chromatography column SP-Sepharose FF, and then subjected to hydrophobic interaction chromatography through a chromatography column Phenyl-Sepharose HP to obtain the Fab fragment of C2H7.
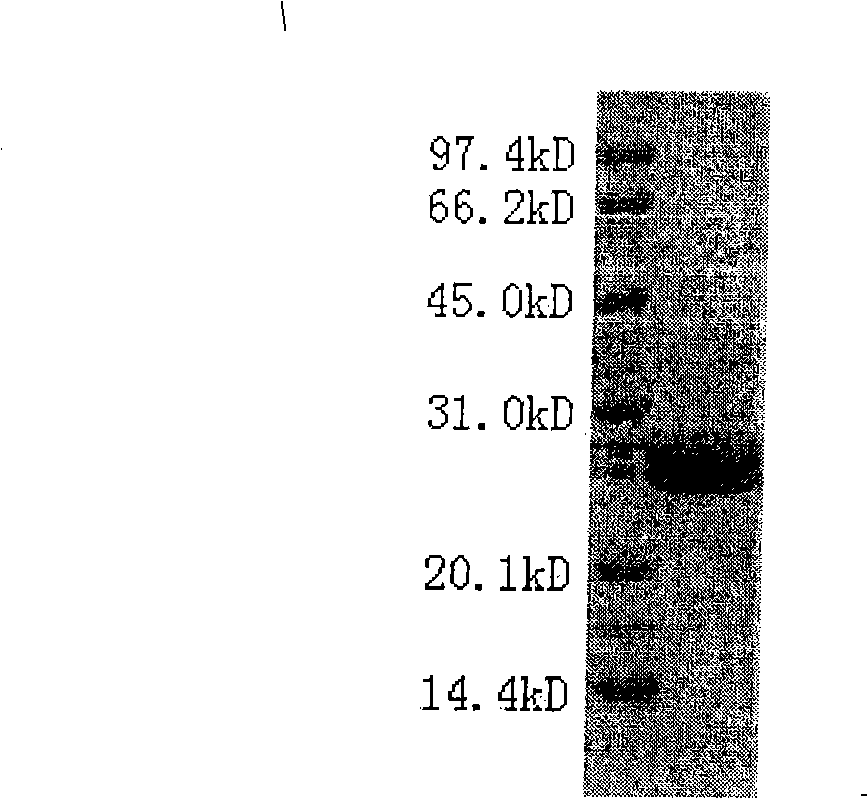
[0025] SDS-PAGE was used to verify the purity and homogeneity of the Fab fragments, and the results were as follows figure 1 shown, according to figure 1 As a result, the purified Fab fragment of C2H7 has a purity greater than 95% and good homogeneity.
[0026] The purified Fab fragment was concentrated to 7 mg / ml, and the buffe...
Embodiment 2
[0027] Example 2 , Preparation of CD20 epitope peptide
[0028] Corresponding to the peptide segment of residues 163 to 187 in the extracellular region of human CD20, an epitope peptide fragment containing 25 amino acids was designed and synthesized. The sequence of the fragment is as follows:
[0029] NIYNCEPANPSEKNSSPSTQYCYSIQ.
[0030] At the same time, an intrachain disulfide bond was introduced between the two cysteines (underlined in the sequence) corresponding to residues 167 and 183 of the extracellular region of human CD20 in this fragment, thereby simulating Out of the natural state of CD20.
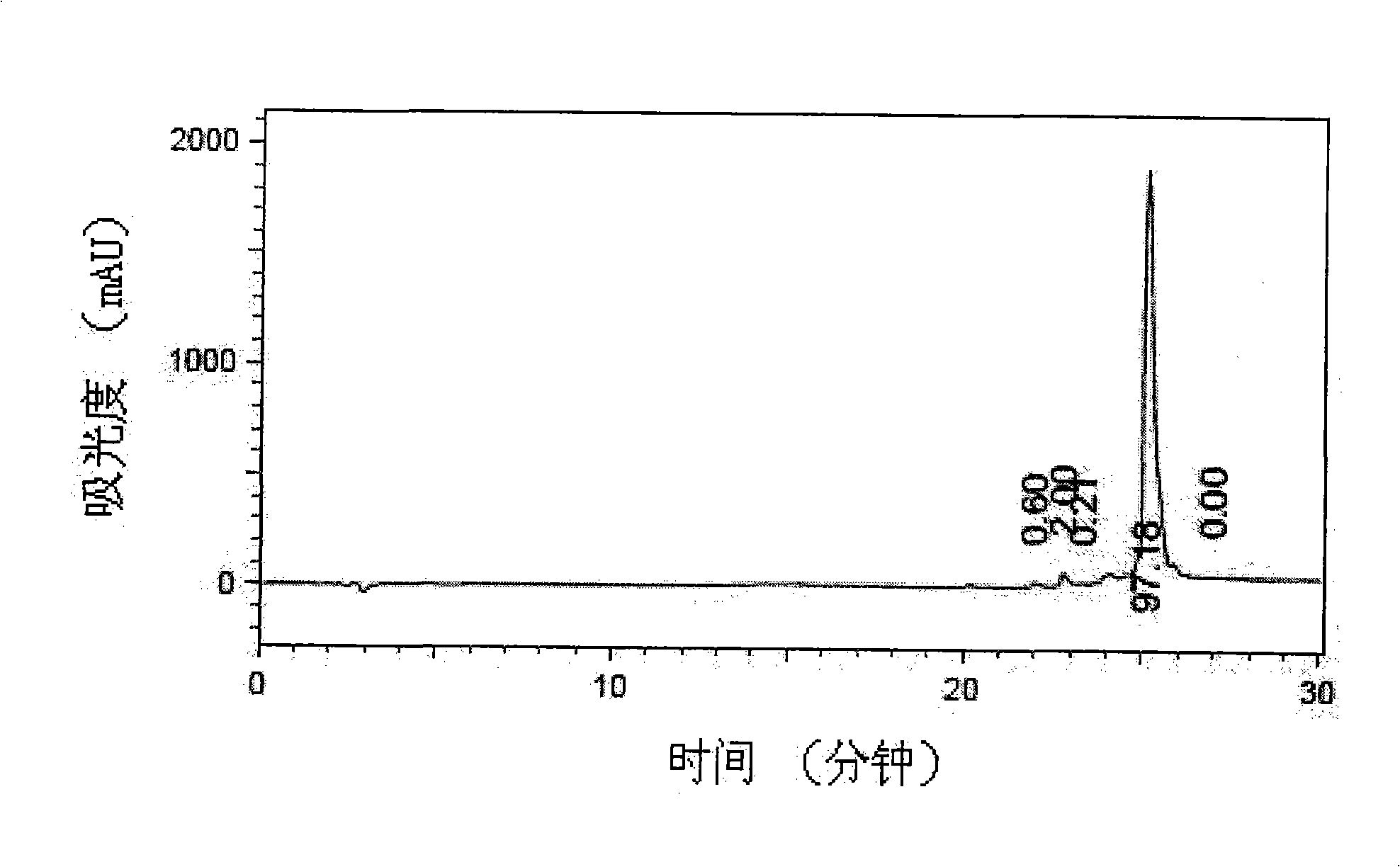
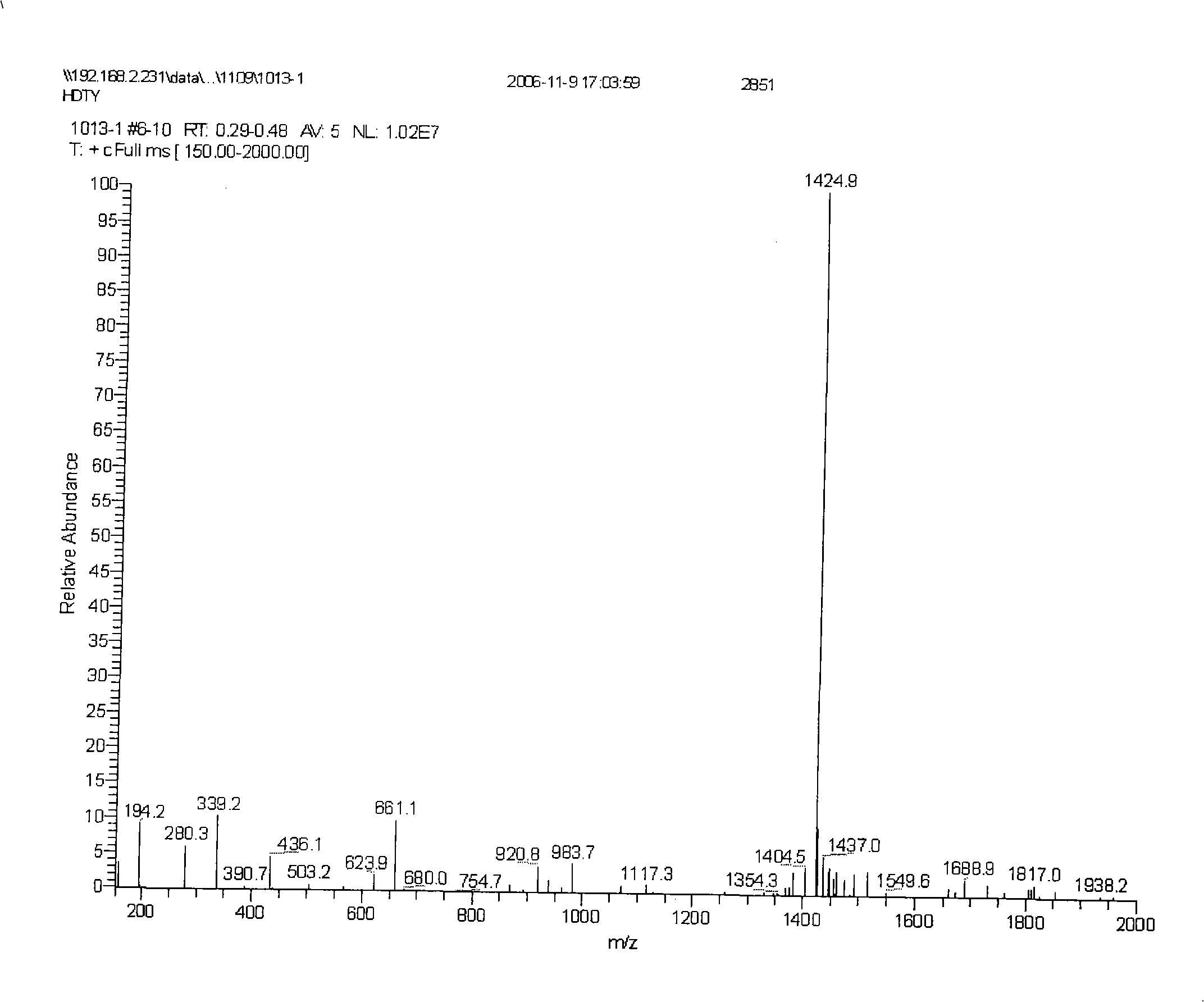
[0031] With reference to the method described in the operation manual, by analytical reversed-phase chromatography ( figure 2 ) and mass spectrometry ( image 3 ), confirming that the purity of the prepared amino acid fragments is greater than 95%.
Embodiment 3-5
[0032] Example 3-5 , Antibody and epitope peptide co-crystallization
[0033] According to the molar ratio of 1:1, 1:5 and 1:10, respectively, the purified C2H7Fab and CD20 epitope peptide were mixed at 4° C. for 12 hours to obtain a protein-peptide mixed solution.
[0034] Using the hanging drop diffusion method, each 1 microliter of the obtained protein-peptide mixed solution was mixed with the crystallization pool liquid (0.2M diammonium hydrogen phosphate, 20% polyethylene glycol 3350) to form a hanging drop, and placed in a Crystal growth was carried out in a crystallization tank with 500 μl of crystallization bath liquid.
[0035] After growing for about two months at 4°C, square co-crystal complex crystals were harvested.
[0036] The crystals were stored frozen in the crystallization pool solution added with 18% polyethylene glycol 500, and then rapidly cooled to -170°C.
[0037] On the BL6A ray source (Japan Photon Factory), carry out the diffraction experiment of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com