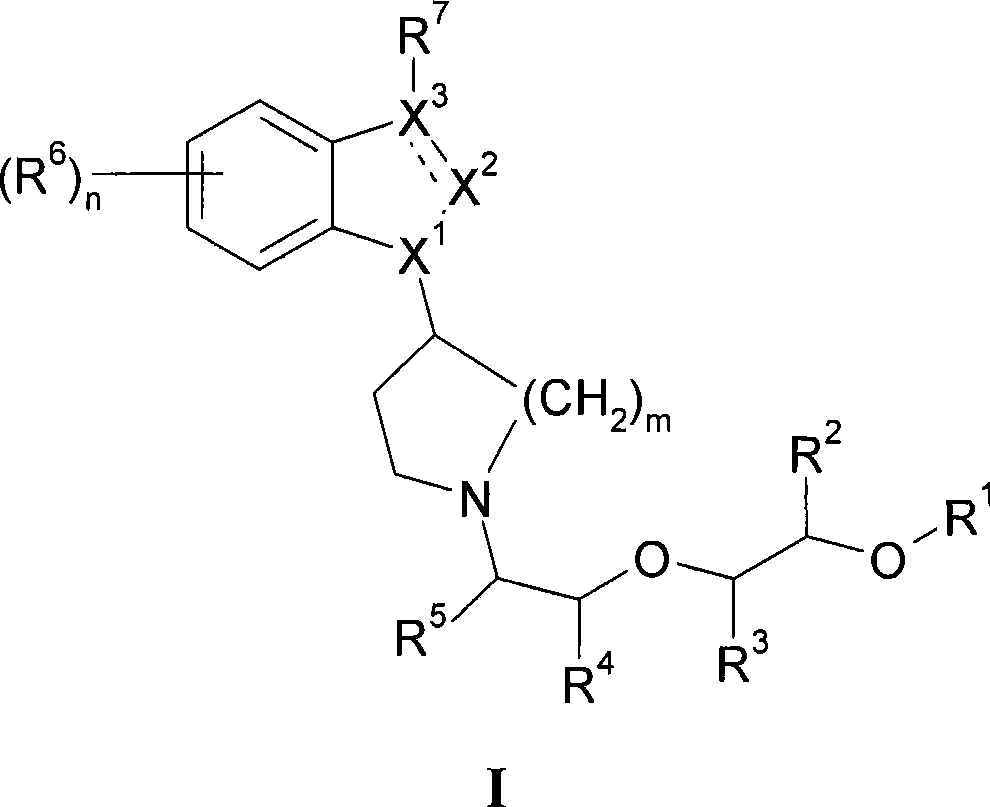
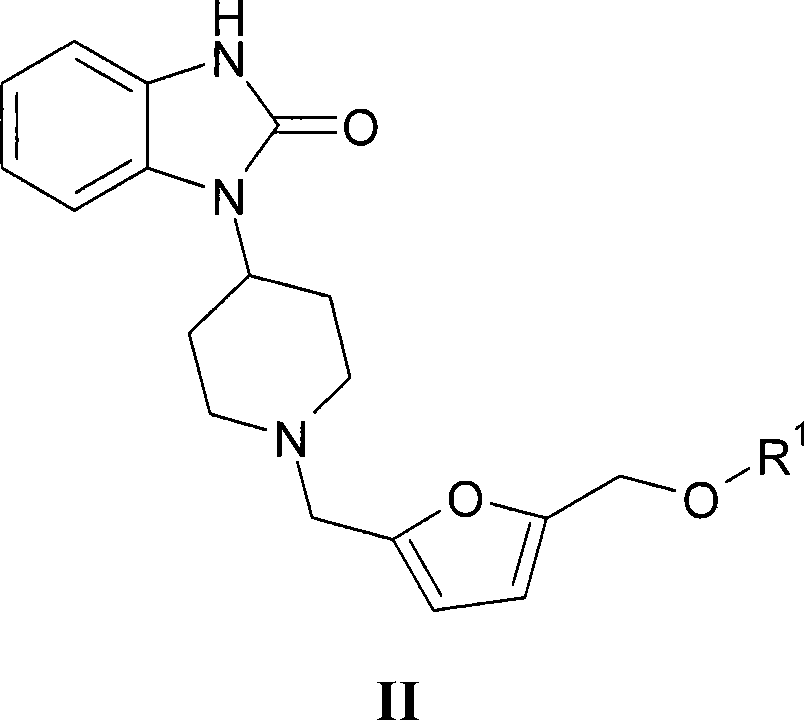
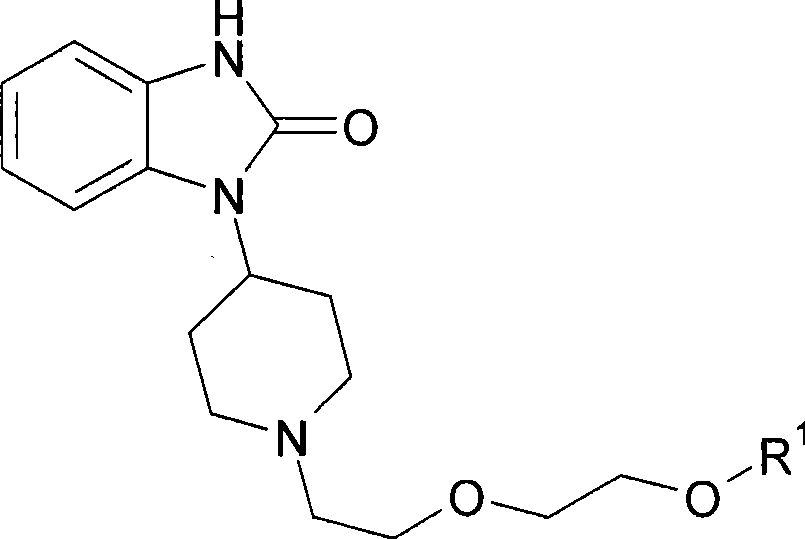
Muscarinic receptor agonists that are effective in the treatment of pain, alzheimer's disease and schizophrenia
A technology for medicinal salts and enantiomers is applied in the field of agonists of muscarinic receptors, and can solve the problems that muscarinic agonists and ACHE-Is are not widely used in clinical practice.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0167] Example 1: 1-(1-{[5-(methoxymethyl)furan-2-yl]methyl}piperidin-4-yl)-1,3-dihydro-2H-benzimidazole- 2-keto
[0168]
[0169]To 1-(piperidin-4-yl)-1,3-dihydro-2H-benzimidazol-2-one (684mg, 3.15mmol), 5-(methoxymethyl)-2-furancarbaldehyde ( 440mg, 3.14mmol) and NaBH (OAc) 3 (680 mg, 3.22 mmol) in dichloromethane (20 mL) was added acetic acid (0.6 mL) dropwise. The mixture was stirred at room temperature for 48 hours. Usual work-up and purification on preparative HPLC gave the title compound which was converted to its HCl salt (620 mg). MS (M+1): 342.08. 1 H NMR (400MHz, methanol-D4): δppm 2.07 (d, J = 13.28Hz, 2H), 2.69-2.92 (m, 2H), 3.19-3.32 (m, 3H), 3.35 (s, 3H), 3.66 (d, J=12.30Hz, 2H), 4.42(s, 2H), 4.46(s, 2H), 4.50-4.62(m, 1H), 6.49(d, J=3.13Hz, 1H), 6.74(d, J=3.13Hz, 1H), 7.01-7.11(m, 3H), 7.29-7.37(m, 1H).
Embodiment 2
[0170] Example 2: 1-{1-[2-(2-methoxyethoxy)ethyl]piperidin-4-yl}-1,3-dihydro-2H-benzimidazol-2-one
[0171]
[0172] 1-Bromo-2-(2-methoxyethoxy)ethane (2.4mmol), 1-(piperidin-4-yl)-1,3-dihydro-2H-benzimidazole-2- A mixture of ketone (2.00 mmol) and sodium carbonate (5 mmol) in acetonitrile (10 mL) was heated at reflux overnight under nitrogen. The reaction mixture was cooled to room temperature, then diluted with diethyl ether (20 mL). The solid was filtered off and the solvent was concentrated. The residue was redissolved in ether (30 mL) and treated with 2N HCl in ether. The precipitate was collected and dried to give the title compound. MS (M+1): 320.05. 1 HNMR (400MHz, DMSO-D6): δ ppm 1.82(d, J=12.50Hz, 2H), 2.80(d, J=12.50Hz, 2H), 3.04-3.31(m, 4H), 3.37-3.71(m, 8H), 3.82(s, 2H), 4.51(t, J=12.11Hz, 1H), 6.96(s, 3H), 7.57(s, 1H), 10.39-11.20(m, 1H), 10.93(s, 1H ).
Embodiment 3
[0173] Example 3: 1-{1-[2-(2-ethoxyethoxy)ethyl]piperidin-4-yl}-1,3-dihydro-2H-benzimidazol-2-one
[0174]
[0175] 1-(piperidin-4-yl)-1,3-dihydro-2H-benzimidazol-2-one (100mg, 0.46mmol), potassium carbonate (250mg, 1.81mmol) and 1-bromo-2- A mixture of (2-ethoxyethoxy)ethane (0.1 mL, 0.64 mmol) in acetonitrile (15 mL) was heated at 50 °C for 48 hours. The mixture was concentrated under reduced pressure. The residue was diluted in dichloromethane, washed with water and dried. The crude product was purified by preparative LCMS (acetonitrile / water) to afford the pure compound as its TFA (trifluoroacetic acid) salt (39%). 1 HNMR (400MHz, chloroform-D): δ ppm 1.16(t, J=7.03Hz, 3H), 1.95(d, J=13.28Hz, 2H), 2.83-3.09(m, 4H), 3.26-3.39(m, 2H), 3.49(q, J=7.03Hz, 2H), 3.52-3.59(m, 2H), 3.58-3.68(m, 2H), 3.78-4.02(m, 4H), 4.52-4.75(m, 1H) , 6.84-7.15 (m, 3H), 7.41 (d, J=7.03Hz, 1H), 10.16 (s, 1H). MS: 334.0 (M+1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com