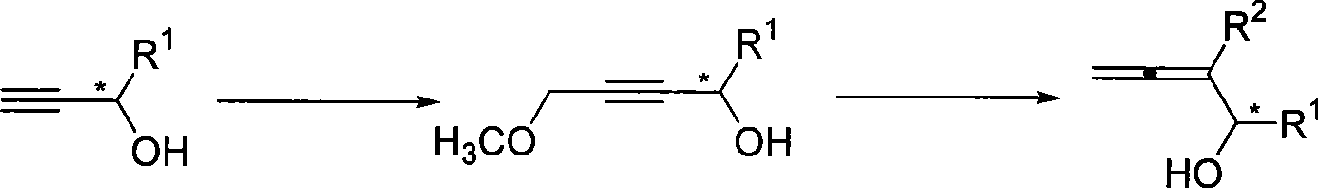
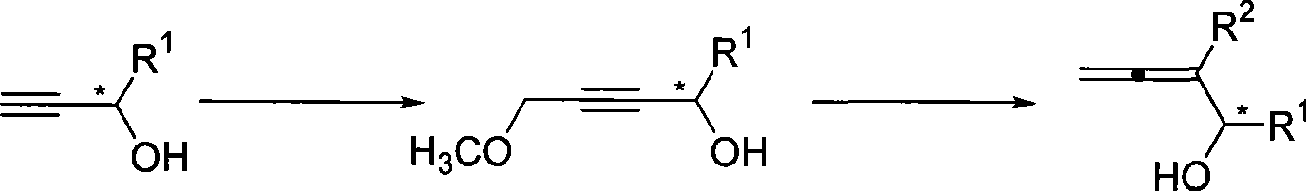
Method for synthesizing optical activity 2,3-allenes secondary alcohol
An optically active, secondary alkenyl alcohol technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of expensive, difficult to obtain reagents or catalysts, etc., and achieve simple operation, convenient mass preparation, and convenient reagents Easy to get effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add tetrahydrofuran (50mL), (S)-1-phenyl-2-propynol (1.3166g, 10.0mmol, enantiomeric excess value 99.6%) to a three-neck flask treated with anhydrous and oxygen-free at room temperature , then dropwise added n-butyllithium (8.8mL, 2.5M inhexanes, 22.0mmol) at -78°C, continued the reaction for half an hour after the dropwise addition, and then added dropwise chloromethyl ether (1.14mL, d=1.06g / mL , 1.21g, 15.0mmol), after reacting for 19 hours, dot the plate to follow the reaction of raw materials completely, add 10mL saturated ammonium chloride solution to quench, extract with 20mL diethyl ether each time, extract three times, wash once with 20mL saturated saline, dry over anhydrous sodium sulfate , filtered, concentrated, and subjected to flash column chromatography to obtain 1.1139 g of the product (R)-4-hydroxy-4-phenyl-2-butynyl methyl ether, with a yield of 63%. The product is a colorless liquid. Product enantiomeric excess: 98.2%, measured by HPLC:
[0020] (HPL...
Embodiment 2
[0022] According to the method described in Example 1, the difference is that the substrate and reagent used are: (R)-1-phenyl-2-propynol (0.6604g, 5.0mmol, enantiomeric excess value 99.9%), normal Butyllithium (4.4mL, 2.5M in hexanes, 11.0mmol) and chloromethyl ether (0.58mL, d=1.06g / mL, 0.615g, 7.5mmol) were reacted in 25mL tetrahydrofuran for 16 hours to obtain the product (S)-4 -Hydroxy-4-phenyl-2-butynyl methyl ether 0.5778g, yield 66%. The product is a colorless liquid. Product enantiomeric excess: 96.1%, measured by HPLC:
[0023] (HPLC condition: Chiralcel AD-H, n-hexane / i-PrOH=90 / 10, 0.8mL / min, λ=230nm, t R 11.7 (major), 13.6 (minor)); [α] 20 D =-16.0° (c=0.70, CHCl 3 ); 1 H NMR (300MHz, CDCl 3 )δ 7.58-7.50(m, 2H), 7.43-7.30(m, 3H), 5.52(d, J=6.0Hz, 1H), 4.19(d, J=1.5Hz, 2H), 3.40(s, 3H) , 2.36 (bs, 1H).
Embodiment 3
[0025] According to the method described in Example 1, the difference is that the substrate and reagent used are: (S)-1-p-methylphenyl-2-propynyl alcohol (0.4642g, 3.2mmol, enantiomeric excess value 99.9% ), n-butyllithium (2.8mL, 2.5M in hexanes, 7.0mmol) and chloromethyl ether (0.36mL, d=1.06g / mL, 0.382g, 4.8mmol) were reacted in 20mL tetrahydrofuran for 14.5 hours to obtain the product (R )-4-hydroxy-4-p-methylphenyl-2-butynyl methyl ether 0.4034 g, yield 67%. The product is a colorless liquid. Product enantiomeric excess: 99.2%, measured by HPLC:
[0026] (HPLC condition: Chiralcel AD-H, n-hexane / i-PrOH=90 / 10, 0.8mL / min, λ=230nm, t r 15.3 (major), 12.7 (minor)); [α] 20 D =+16.4° (c=0.80, CHCl 3 ); 1 HNMR (300MHz, CDCl 3 )δ 7.43(d, J=8.1Hz, 2H), 7.19(d, J=8.1Hz, 2H), 5.49(d, J=6.3Hz, 1H), 4.19(d, J=1.5Hz, 2H), 3.40(s, 3H), 2.36(s, 3H), 2.21(d, J=6.3Hz, 1H); 13 C NMR (75MHz, CDCl 3 )δ 137.9, 137.6, 129.0, 126.4, 86.6, 81.6, 64.0, 59.7, 57.4, 21.0; IR (neat) v (cm ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com