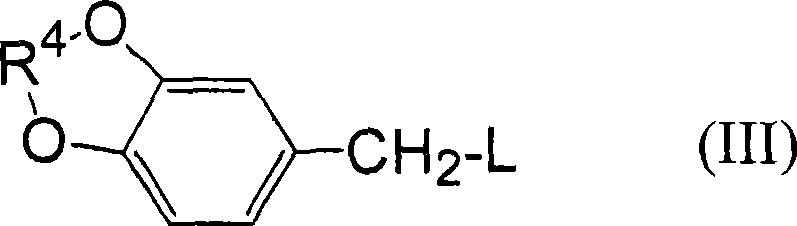Process for production of benzaldehyde co.mpound
A compound and technology of benzaldehyde, applied in the field of benzaldehyde compounds, can solve the problems of increasing environmental load, complicated operation and difficulty, and achieve the effect of reducing the amount of production and reducing the environmental load
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
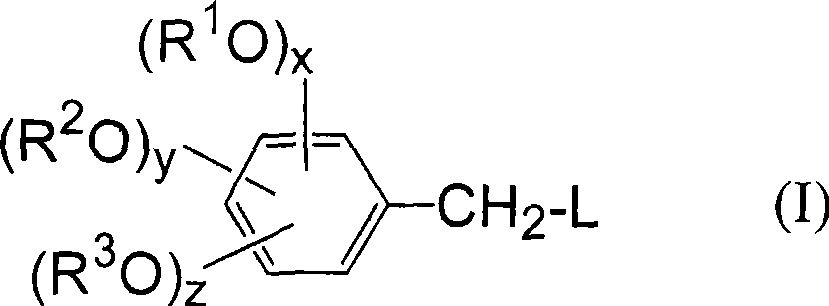
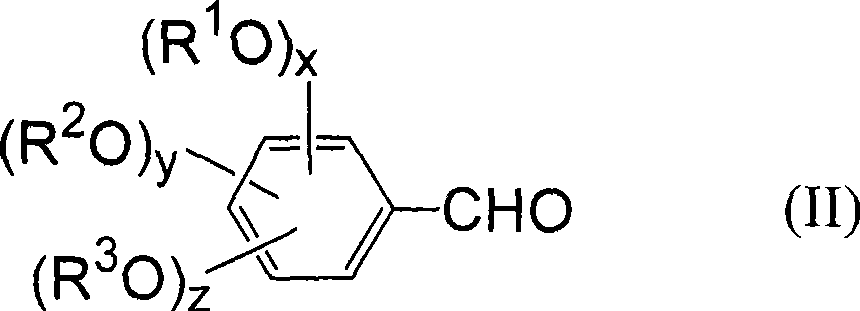
[0041] According to the production method of the benzaldehyde compound of the present invention, the benzyl compound having a leaving group used as a starting material is represented by the following general formula (I).
[0042]
[0043] In the above formula (I), L represents a group selected from a halogen atom, a hydroxysulfonyloxy group, an alkylsulfonyloxy group with or without a substituent, and an arylsulfonyloxy group with or without a substituent. Leaving group;
[0044] x, y, and z are the number of alkoxy substituents, each representing an integer of 0 or 1, and combined according to x+y+z becoming an integer of 1 to 3; R 1 ~R 3 Each independently represents a hydrogen atom, or a hydrocarbon group with or without a substituent, or, when x+y+z represents an integer of 2 or 3, an alkoxy substituent: OR 1 , OR 2 , OR 3 Any two of them can be bonded to each other to form an alkylenedioxy group, and form a ring structure together with two carbon atoms adjacent to ...
Embodiment 1
[0109] Example 1 (Synthesis of piperonal [heliotropin heliotropin]: molar ratio of hexamethylenetetramine to benzyl chloride compound: 0.25)
[0110] In a 200ml 3-necked flask, mix 17.06g (100mmol) of piperonyl chloride and 8.5ml of acetic acid with a purity of more than 96%, and add 3.50g (25mmol) of hexamethylenetetramine at a temperature of 20 to 27°C. , at a temperature of 115-125° C., stirring while refluxing to react, and prepare a mixed solution containing piperonyl chloride / hexamethylenetetramine salt (1). The mixed solution (1) was refluxed at a temperature of 115 to 125° C., and the mixed solution formed by 8.5 ml of water and 6.3 ml of hydrochloric acid aqueous solution with a concentration of 35% by mass was added dropwise thereto, to the mixed reaction solution obtained (pH: 0.80) at a temperature of 90 to 100° C. and stirred under reflux for 2 hours to synthesize piperonal. After the above reaction was completed, the obtained reaction solution was left to cool t...
Embodiment 2
[0115] Embodiment 2 (the synthesis of piperonal: the mol ratio of hexamethylenetetramine to benzyl chloride compound: 0.39)
[0116] In a 200ml 3-neck flask, mix 114.6g (1.10mol) of aqueous hydrochloric acid solution with a concentration of 35% by weight and 9.79g (0.300mol) of paraformaldehyde with a purity of 92% by weight, and cool the flask to an internal temperature of 8-9°C . A solution of 12.21 g (0.100 mol) of 1,2-methylenedioxybenzene in 20 ml of toluene was slowly added dropwise to the above liquid mixture, and stirred for 7 hours while maintaining the internal temperature at 8 to 9°C to allow reaction. After the reaction was completed, the obtained reaction solution was transferred to a separatory funnel, and the water layer was separated and removed. The organic layer obtained is transferred in the 3-neck flask of 200ml, after toluene is distilled off under reduced pressure, the concentrate obtained is carried out quantitative analysis with gas chromatography (int...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com