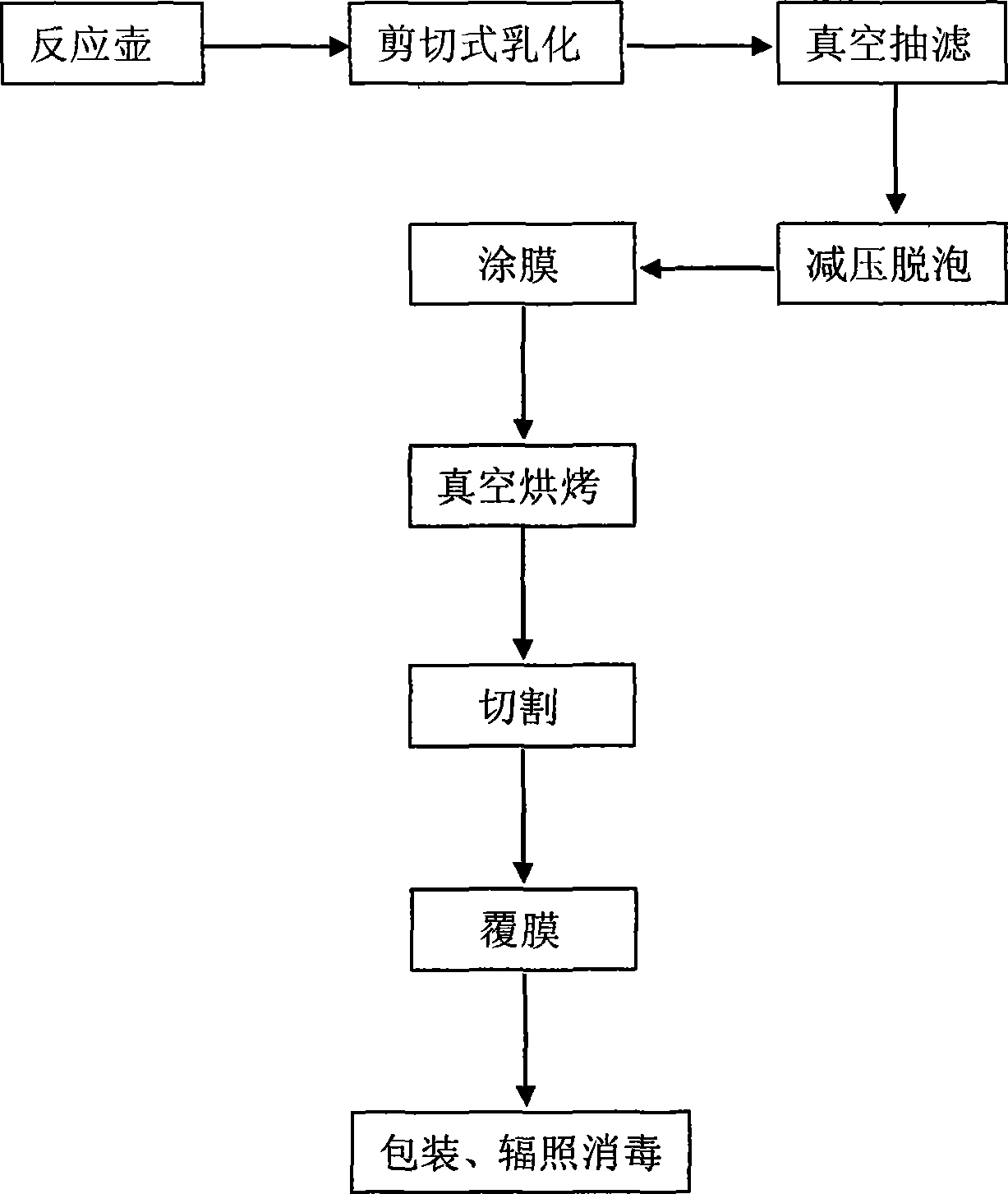
Dressing production process
A production process and defoaming technology, which is applied in the field of medical supplies, can solve the problems of no support for the drug film, long baking time, and difficult control, and achieve the effects of easy large-scale production, shortened production time, and improved product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Get 2Kg of carboxymethyl chitosan, add 60Kg of water to fully mix, then use a shear emulsifier to stir at 20°C for 2 hours to make it evenly mixed, and the pressure is 5 × 10 5 Vacuum filtration under the condition of Pa, the pressure is 7×10 5 Degassing under reduced pressure under the condition of Pa; (2) the solution after degassing is poured into the mould with Teflon anti-sticking coating and coated into film; (3) the mould that is coated is put into vacuum oven at Bake at 60°C for 3 hours, and obtain a carboxymethyl chitosan film after demoulding; (4) use a step-by-step laminating machine to cover one side of the carboxymethyl chitosan film with non-woven fabric or polyurethane film, and the other side Cover with release paper, then cut, pack, and sterilize.
Embodiment 2
[0028] (1) Get 1.5Kg of carboxymethyl chitosan, add 75Kg of water to fully mix, and then use a shearing emulsifier to stir for 1 hour at 30°C to make it evenly mixed. 5 Vacuum filtration under the condition of Pa, the pressure is 9×10 5 Degassing under reduced pressure under the condition of Pa; (2) Pour the solution after degassing into the mold and coat it to form a film; (3) Put the coated mold into a vacuum oven and bake it at 40°C for 5 hours, and demold Finally obtain carboxymethyl chitosan film; (4) use step-by-step laminating machine to cover non-woven fabric or polyurethane film on one side of carboxymethyl chitosan film, and the other side covers release paper, and then it Cut, pack, sterilize.
[0029] In the embodiment of the present invention, carboxymethyl chitosan is used as raw material, and sodium alginate, sodium carboxymethyl cellulose, a mixture of carboxymethyl chitosan and sodium alginate or other drugs capable of promoting wound healing can also be used...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com