Preparation and utility of substituted carboxylic acid compounds
A compound and mixture technology, applied in the fields of application, anhydride/acid/halide active ingredients, botany equipment and methods, etc., can solve problems such as adverse events and complex applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
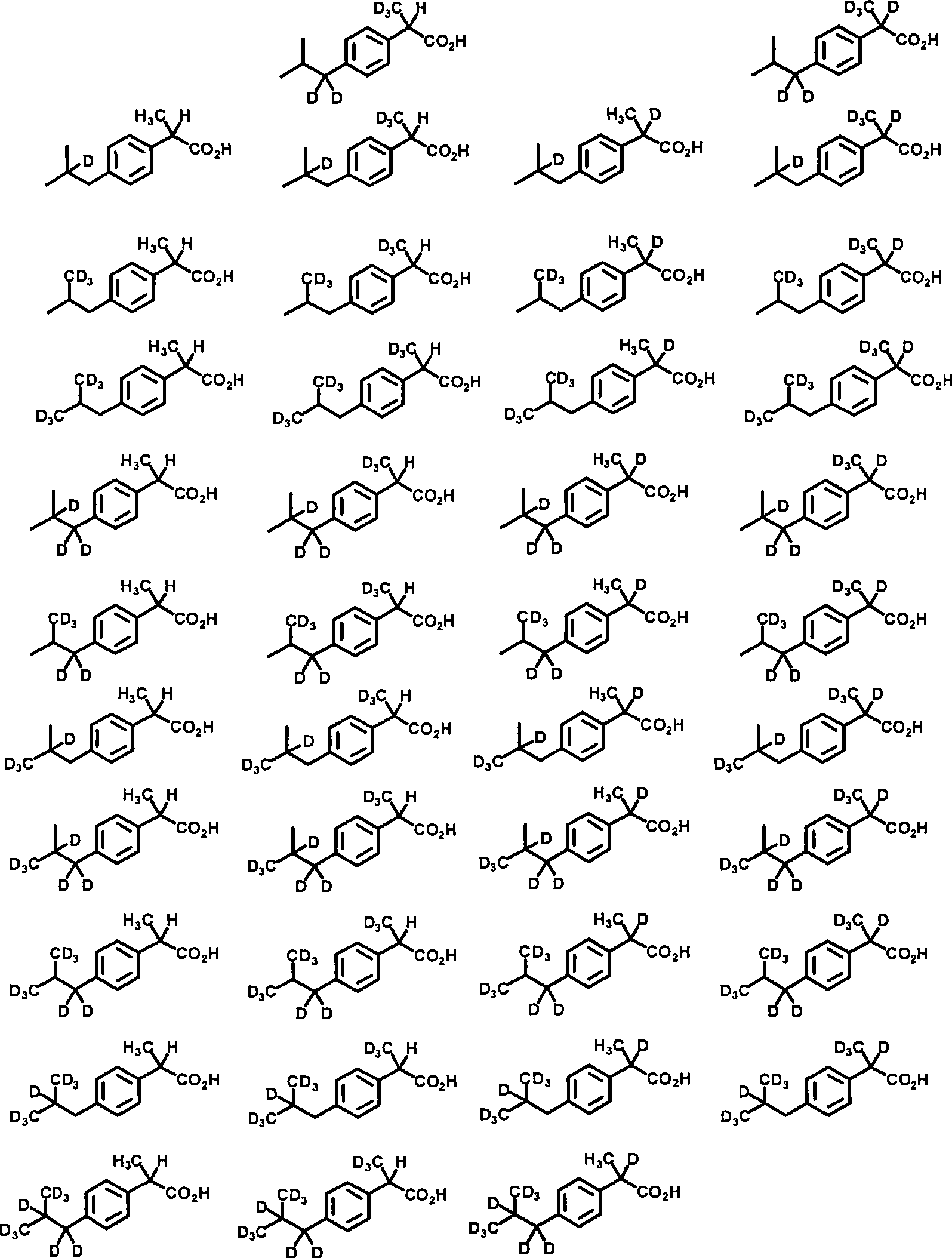
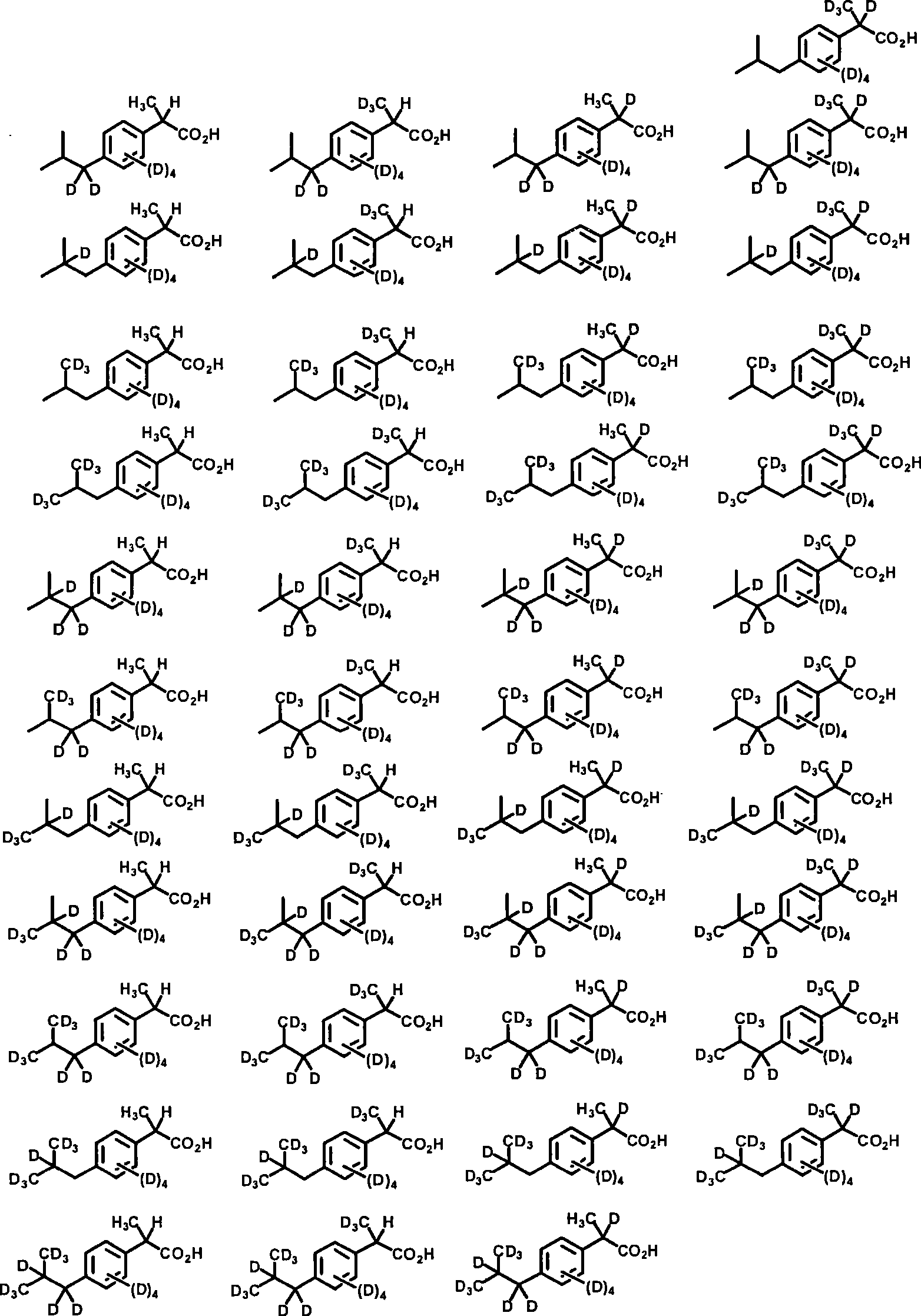
Embodiment 1
[0322] d 13 -2-(4-Isobutyl-phenyl)-propionic acid (d 13 - ibuprofen)
[0323]
[0324] D in thick wall pressure pipe 2 A mixture of ibuprofen (206 mg, 1.00 mmol) in O (5 mL) was treated with 10% Pd / C (20 mg) and washed with N 2 Bubble deoxygenation. Sodium formate (102 mg, 1.5 equiv) was added and the tube was then capped and surrounded by a blast shield. The temperature was raised to 160°C and held for 24 hours. After cooling to ambient temperature, the tube was uncapped, then the reaction mixture was diluted with diethyl ether, and 1 N HCl (2 eq.) was added. Separate the organic layer, anhydrous MgSO 4 Drying and concentration under reduced pressure gave a white solid (163 mg). This operation was repeated for 143 mg of the above product to give a white solid (127 mg, 66% for two steps). 1 H NMR (300MHz, CDCl 3 , 4-chloro-1-methoxy-2-nitrobenzene was used as internal standard) 7.12 (d, 2H), 7.23 (d, 2H).
Embodiment 2
[0326] d 17 -2-(4-Isobutyl-phenyl)-propionic acid (d 17 - ibuprofen)
[0327]
[0328] Prepared according to Sajiki, 2005. d in a closed tube under hydrogen pressure in the presence of 5% Pt / C (20 wt% of substrate) 13 - ibuprofen in D 2 O heated to 180 ° C.
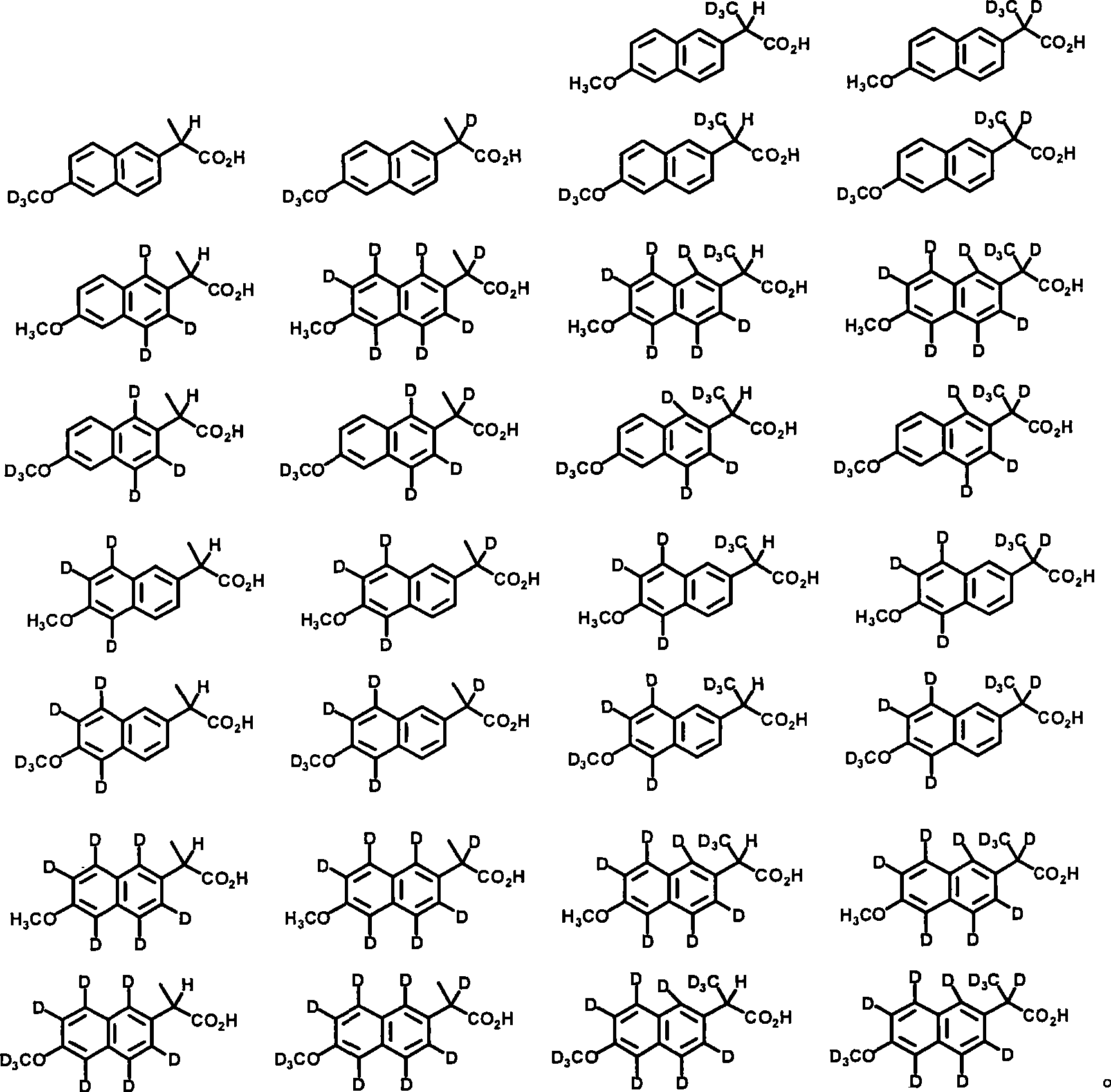
Embodiment 3
[0330] 2-(6-Hydroxynaphthalen-2-yl)-propionic acid
[0331]
[0332] A mixture of naproxen (1.15 g, 5.00 mmol) in glacial acetic acid (10 mL) was treated with 48% aqueous HBr (2.5 mL) and heated to 150 °C for 3 hours. Most of the solvent and acid were distilled off, and the mixture was cooled to room temperature. The resulting solid was washed with H 2 O (20 mL), stirred for 30 minutes, filtered, and washed with H 2 Washed with O and hexane, and dried under reduced pressure to give an off-white solid (1.038 g, 4.80 mmol, 96%). 1 H NMR (300MHz, acetone-d 6 ) 1.63 (d, 3H), 3.62 (s, 3H), 3.90 (q, 2H), 7.18 (m, 2H), 7.41 (m, 1H), 7.67 (m, 1H), 7.78 (m, 2H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com