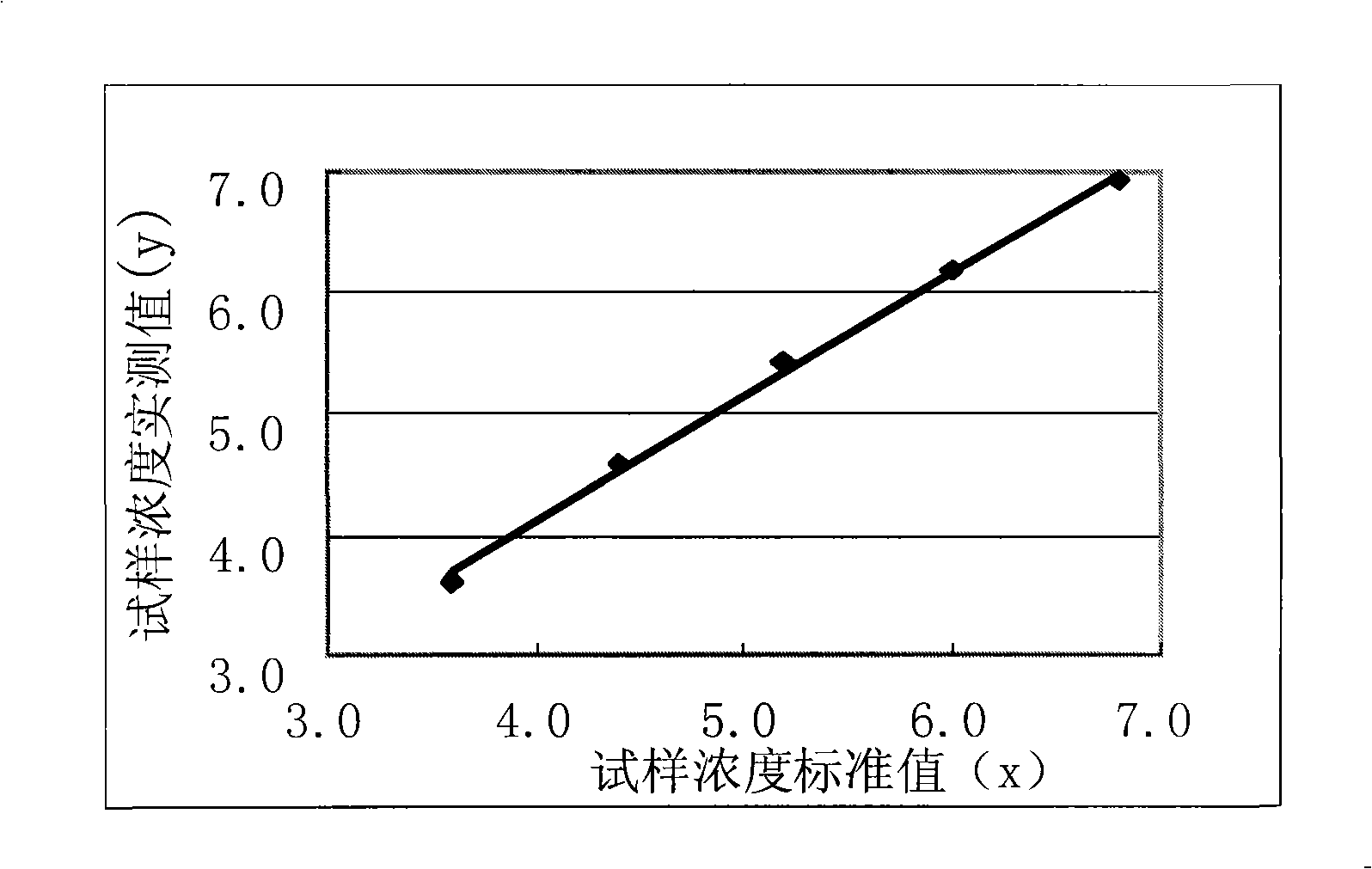
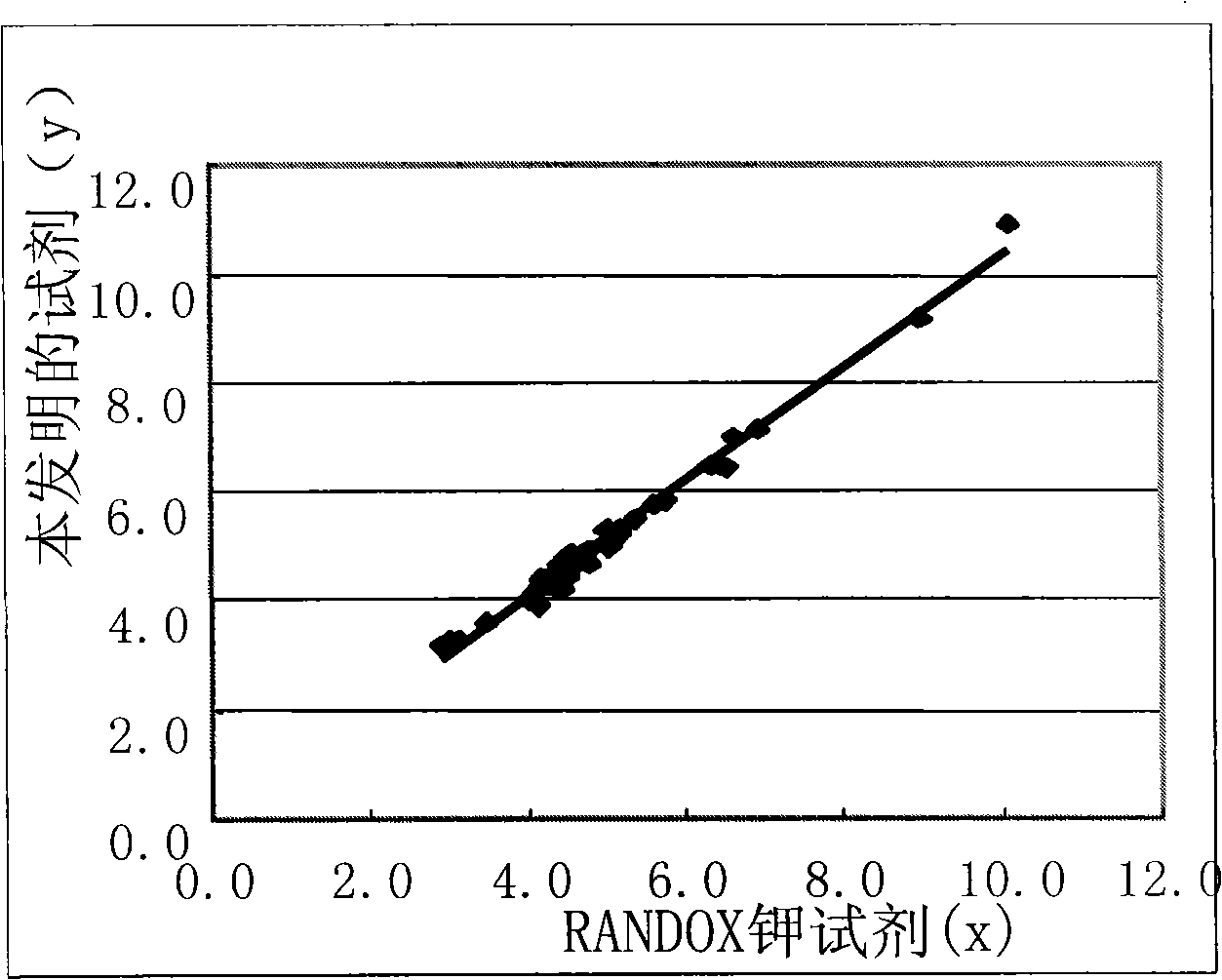
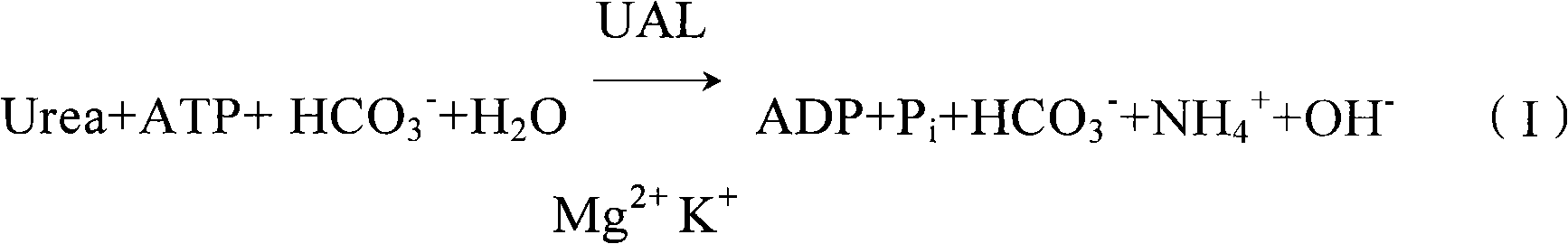
Clinic in vitro potassium determination reagent by enzyme method
A technology of in vitro enzymes and reagents, applied in the determination/testing of microorganisms, biochemical equipment and methods, etc., can solve the short-term problems, such as replacement once every three months or so
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0031] The present invention will be described in further detail below in conjunction with specific embodiments.
[0032] The clinical in vitro enzymatic potassium determination reagent of the present invention comprises components of the following concentrations: 0.10-0.40u / ml urea aminohydrolase UAL, 20.0-80.0u / ml glutamate dehydrogenase GLDH, 20.0-40.0mmol / L urea Urea, 1.0-3.0mmol / L adenosine triphosphate ATP, 10.0-15.0mmol / L sodium bicarbonate NaHCO 3 , 5.0-10.0mmol / L magnesium sulfate MgSO 4 , 10.0-20.0mmol / L α-ketoglutarate, 0.30-0.45mmol / L reduced coenzyme I, 0.30-0.50mmol / L ethylene glycol bis(2-aminoethyl ether)tetraacetic acid EGTA, 0.1~0.2 g / L sodium azide NaN 3 .
[0033] In the actual production process, the clinical in vitro diagnostic potassium determination reagent of the present invention is a double reagent, comprising a first reagent part (R1) and a second reagent part (R2), urea aminohydrolase hydrolase, glutamic acid dehydrogenase, Urea, adenosine tri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com