Substituted acylanilides and methods of use thereof
A technology for medicinal products and compounds, applied to substituted N-acyl anilides and their application fields, can solve the problems of increasing the risk of foot ulcers, complicating problems and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0303] The preparation of pharmaceutical compositions comprising active ingredients is known in the art, for example by mixing, granulating or tabletting methods. The active therapeutic ingredient is usually mixed with excipients which are pharmaceutically acceptable and compatible with the active ingredient. For oral administration, the compounds according to the invention or their physiologically tolerated derivatives such as salts, esters, N-oxides etc. are mixed with additives customary for this purpose such as carriers, stabilizers or inert diluents, and Conversion into a form suitable for administration, such as tablets, coated tablets, hard or soft gelatin capsules, aqueous, alcoholic or oily solutions, is carried out by conventional methods. For parenteral administration, the compounds of the present invention or their physiologically tolerated derivatives such as salts, esters, N-oxides, etc. are converted into solutions, suspensions or emulsions, if necessary, with c...
Embodiment 5
[0600] Example 5 demonstrates that the compounds of formula (I) have anabolic activity and minimal androgenic activity, and therefore these compounds can be used in the treatment of patient populations who were contraindicated in the past androgen. Compounds of formula (I) have been shown to stimulate muscle growth regardless of the presence or absence of testosterone and still exert an antiproliferative effect on the prostate, thus in one embodiment the methods of the invention restore muscle mass loss in sarcopenia or cachexia patients.
[0601] In one embodiment, the compounds described herein alter leptin levels in a subject. In another embodiment, the compounds described herein lower leptin levels. In another embodiment, the compounds described herein increase leptin levels in a subject. Leptin is known to have an effect on appetite in obese mice, resulting in weight loss, so it is associated with obesity.
[0602] In one embodiment, the compounds described herein affec...
Embodiment 1
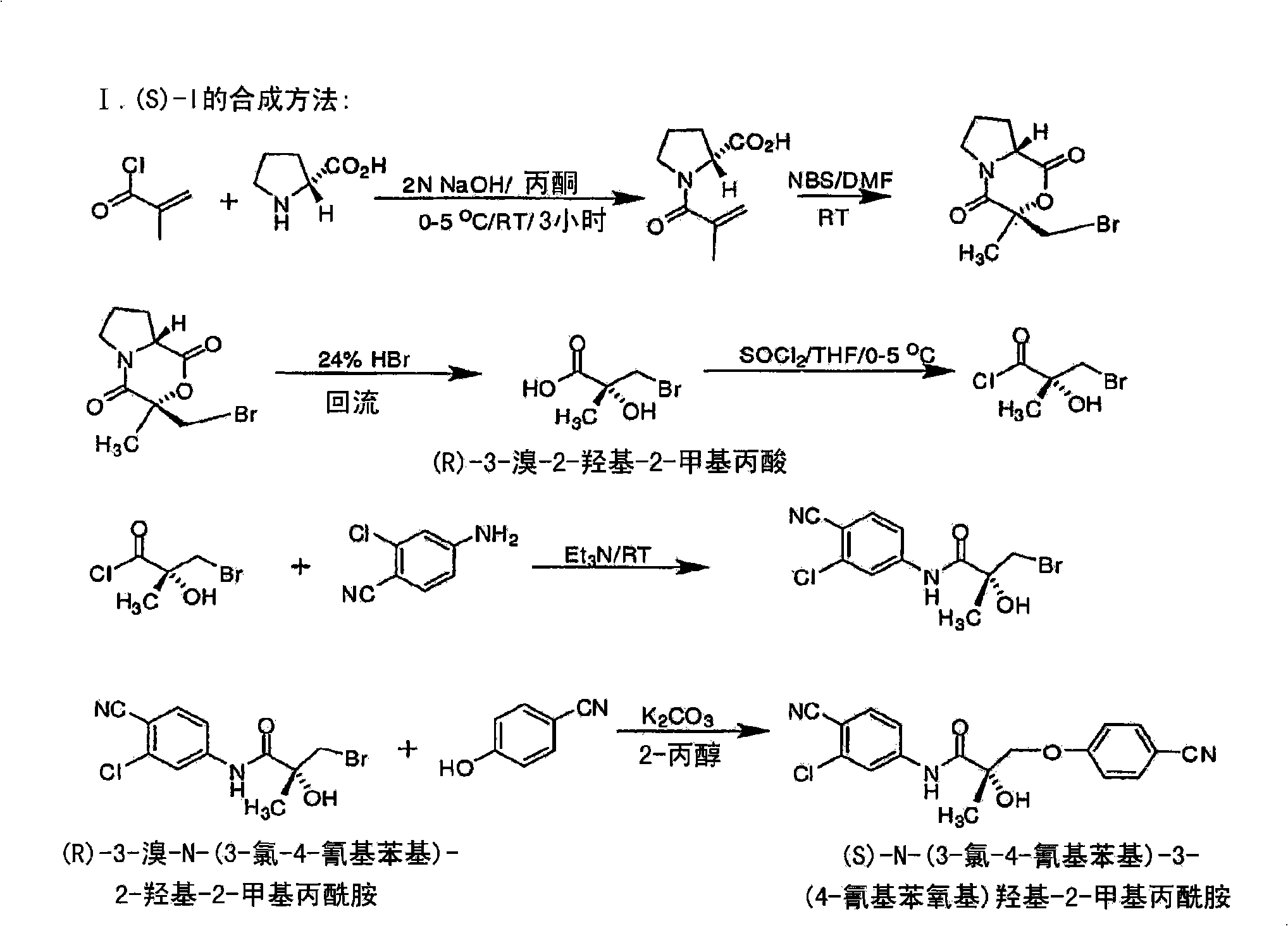
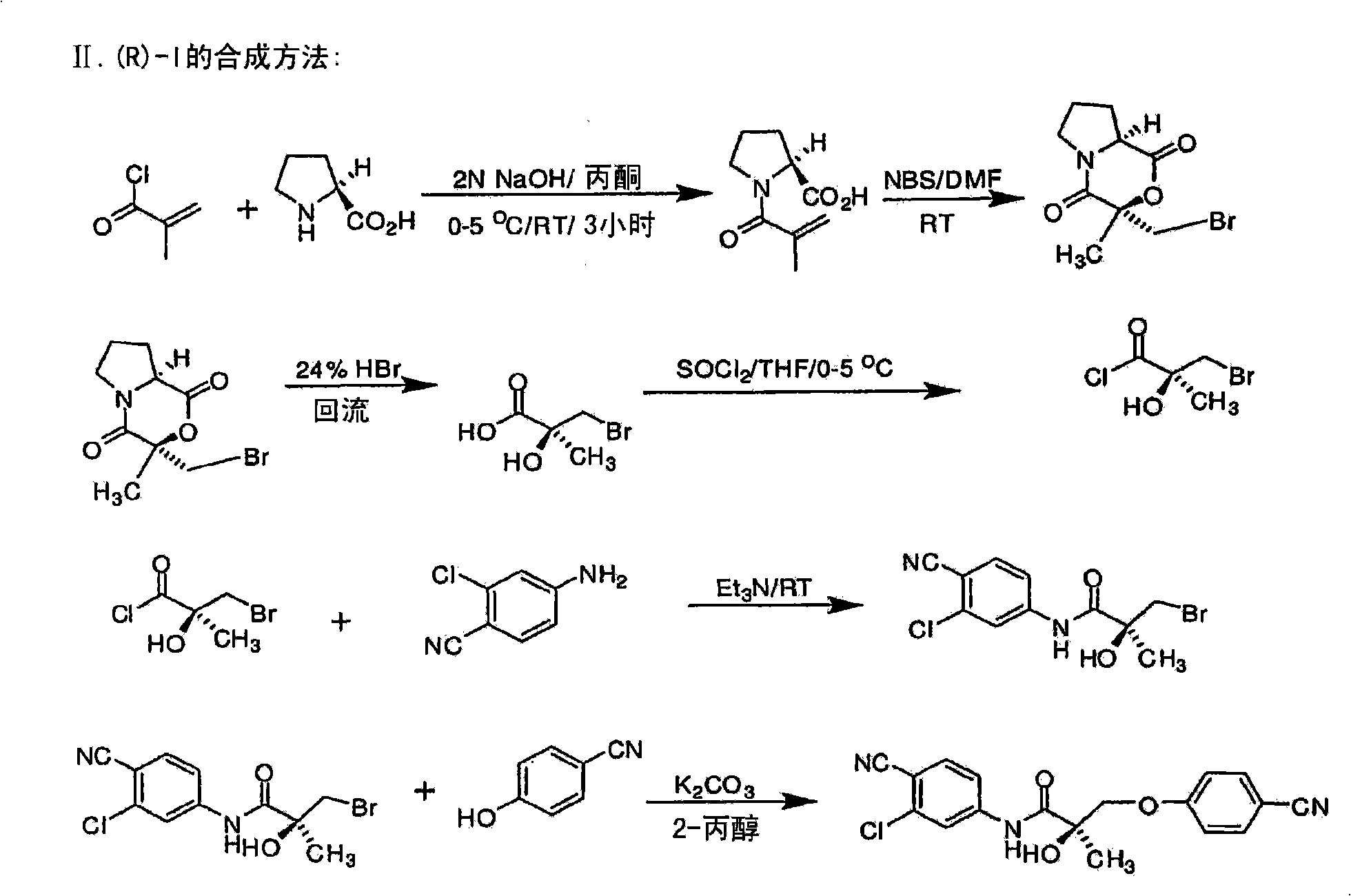
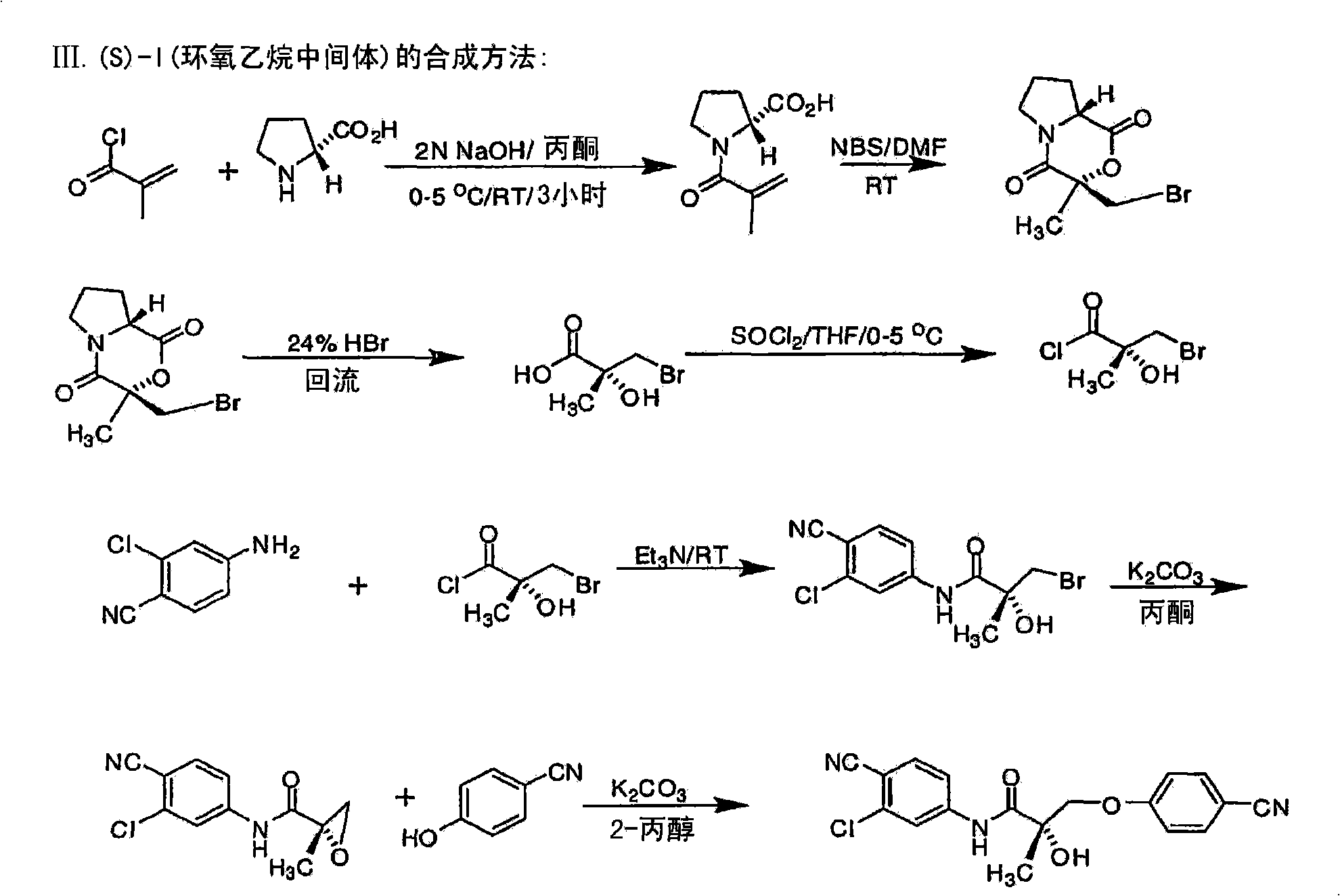
[0683] Synthesis of the (S) enantiomer of the compound of formula (I) ( Figures 1A-1L )
[0684]
[0685] (2R)-1-methacryloylpyrrolidine-2-carboxylic acid. D-proline (14.93g, 0.13mol) was dissolved in 71mL of 2N NaOH and cooled in an ice bath; the resulting alkaline solution was washed with acetone (71mL )dilution. A solution of methacryloyl chloride (13.56 g, 0.13 mol) in acetone (71 mL) and 2N NaOH (71 mL) were added simultaneously to an aqueous D-proline solution in an ice bath over 40 min. During the addition of methacryloyl chloride, the pH of the mixture was maintained at 10-11°C. After stirring (3 h, room temperature), the mixture was evaporated in vacuo at a temperature of 35-45 °C to remove acetone. The resulting solution was washed with ether and acidified to pH 2 with concentrated HCl. The acidic mixture was saturated with NaCl and extracted with EtOAc (100 mL x 3). Combined extracts with Na 2 SO 4 Dry, filter through Celite, and evaporate in vacuo to g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com