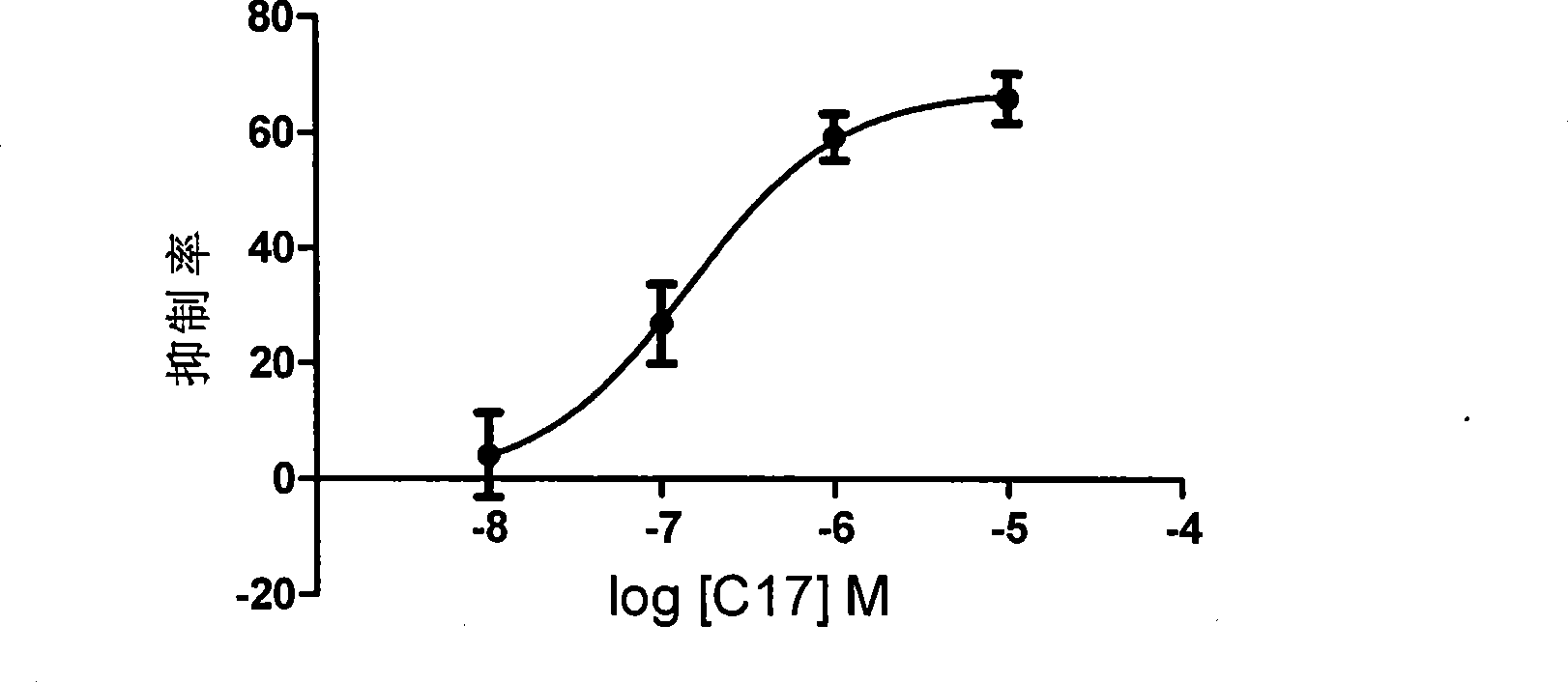
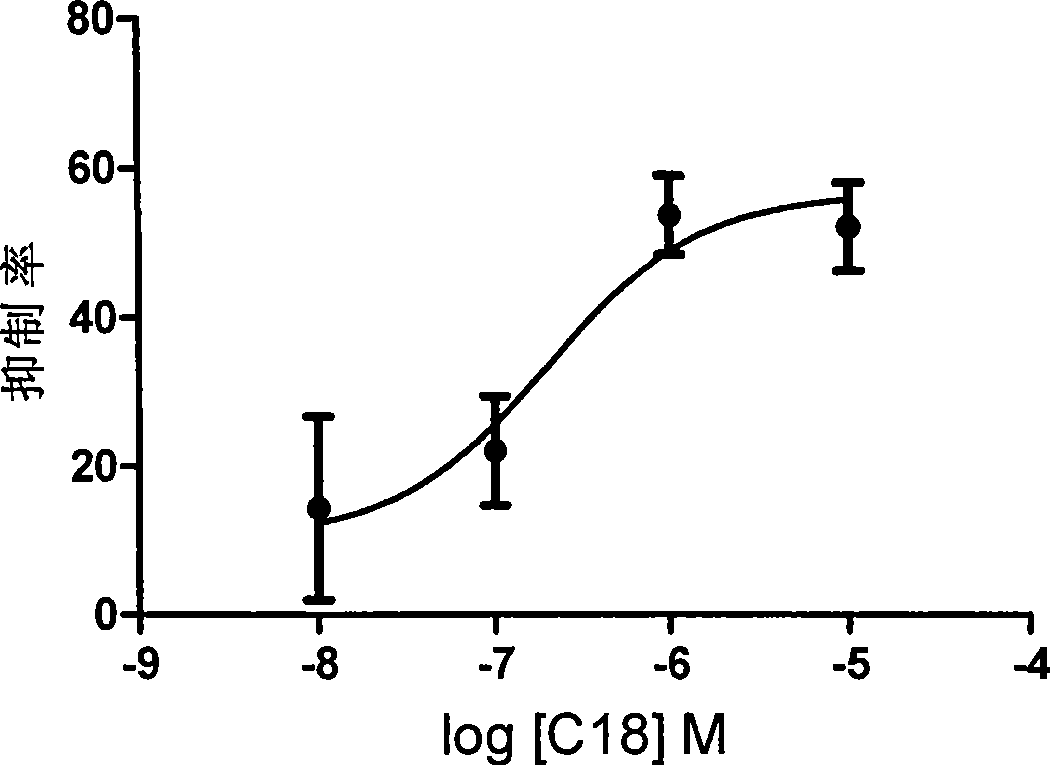
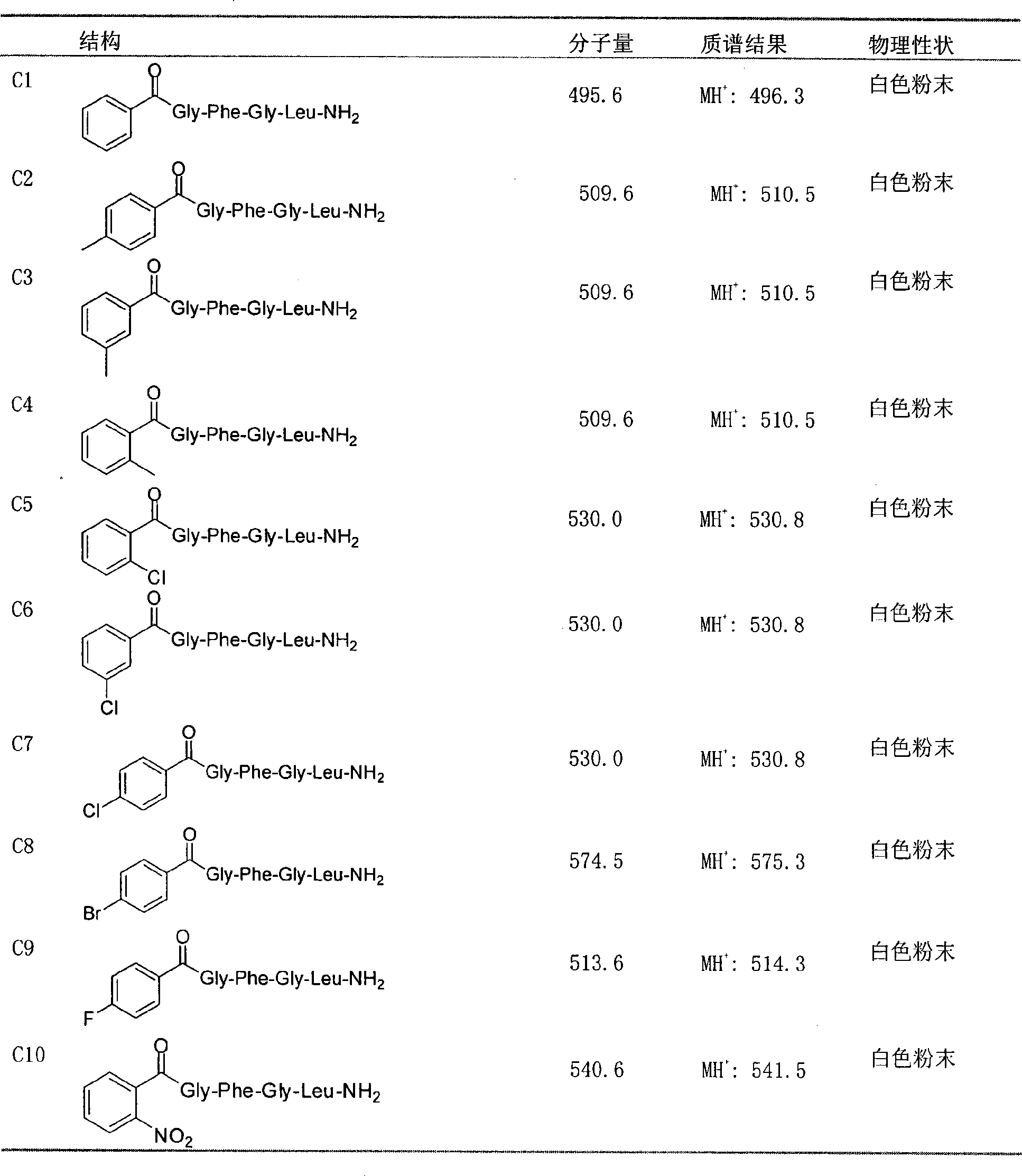
Novel pentapeptide analogue and application thereof
A technology of analogues and drugs, applied in the field of new pentapeptide analogues, can solve the problems of unsatisfactory in vivo effects, unsatisfactory in vivo activity, high synthesis cost, etc., achieve good development and application prospects, and inhibit the in vitro activity of juvenile hormone synthesis , the effect of simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0024] The present invention can be further described in detail by the following examples, but the present invention is not limited to these examples. Synthesis of Preparation Example 1, C1 compound
[0025] (1) Resin activation: Weigh 200mg Rink Amide-AM resin, wash 4 times with DCM, add 15ml DCM to swell and activate for 3 hours, wash 4 times with DMF, add 20% piperidine DMF solution to cut for 20min, wash 4 times with 5ml DMF, 5ml DCM washed 4 times, Kaiser's reagent detection.
[0026] (2) Connect with Leu: DMF wash 3 times, add 106mg Fmoc-Leu-OH (3 times the amount), 114mg HBTU (3 times the amount), 41mg HOBt (3 times the amount), 105ul DIEA (6 times the amount), dissolve in 15ml DMF, stirred at room temperature for 2h, washed 4 times with 5ml DMF, cut by adding 20% piperidine DMF solution for 20min, washed 4 times with 5ml DMF, washed 4 times with 5ml DCM, detected by Kaiser's reagent.
[0027] (3) Connect to Gly: wash 3 times with DMF, add 90mg Fmoc-Gly-OH, 114mg HB...
preparation Embodiment 2、C14
[0034] Synthesis of Preparation Example 2, C14 Compound
[0035] (1) Resin activation: Weigh 200mg Rink Amide-AM resin, wash 4 times with DCM, add 15ml DCM to swell and activate for 3 hours, wash 4 times with DMF, add 20% piperidine DMF solution to cut for 20min, wash 4 times with 5ml DMF, 5ml DCM washed 4 times, Kaiser's reagent detection.
[0036] (2) Connect with Leu: DMF wash 3 times, add 106mg Fmoc-Leu-OH (3 times the amount), 114mg HBTU (3 times the amount), 41mg HOBt (3 times the amount), 105ul DIEA (6 times the amount), dissolve in 15ml DMF, stirred at room temperature for 2h, washed 4 times with 5ml DMF, cut by adding 20% piperidine DMF solution for 20min, washed 4 times with 5ml DMF, washed 4 times with 5ml DCM, detected by Kaiser's reagent.
[0037] (3) Connect to Gly: wash 3 times with DMF, add 90mg Fmoc-Gly-OH, 114mg HBTU, 41mg HOBt, 105ul DIEA, dissolve in 15ml DMF, stir at room temperature for 2h, wash 4 times with 5ml DMF, add 20% piperidine DMF solution Cu...
preparation Embodiment 3、C15
[0044] Synthesis of Preparation Example 3, C15 Compound
[0045] (1) Resin activation: Weigh 200mg Rink Amide-AM resin, wash 4 times with DCM, add 15ml DCM to swell and activate for 3 hours, wash 4 times with DMF, add 20% piperidine DMF solution to cut for 20min, wash 4 times with 5ml DMF, 5ml DCM washed 4 times, Kaiser's reagent detection.
[0046] (2) Connect with Leu: DMF wash 3 times, add 106mg Fmoc-Leu-OH (3 times the amount), 114mg HBTU (3 times the amount), 4lmg HOBt (3 times the amount), 105ul DIEA (6 times the amount), dissolve in 15ml DMF, stirred at room temperature for 2h, washed 4 times with 5ml DMF, cut with 20% piperidine DMF solution for 20min, washed 4 times with 5ml DMF, 4 times with 5ml DCM, detected by Kaiser's reagent.
[0047] (3) Connect to Gly: wash 3 times with DMF, add 90mg Fmoc-Gly-OH, 114mg HBTU, 41mg HOBt, 105ul DIEA, dissolve in 15ml DMF, stir at room temperature for 2h, wash 4 times with 5ml DMF, add 20% piperidine DMF solution Cut for 20min, w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com