Process for production of dialkyltin dialkoxides
A manufacturing method and technology of dialkyl tin, applied in chemical instruments and methods, tin organic compounds, compounds of elements of Group 4/14 of the periodic table, etc. Carbonate cost and unresolved waste issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
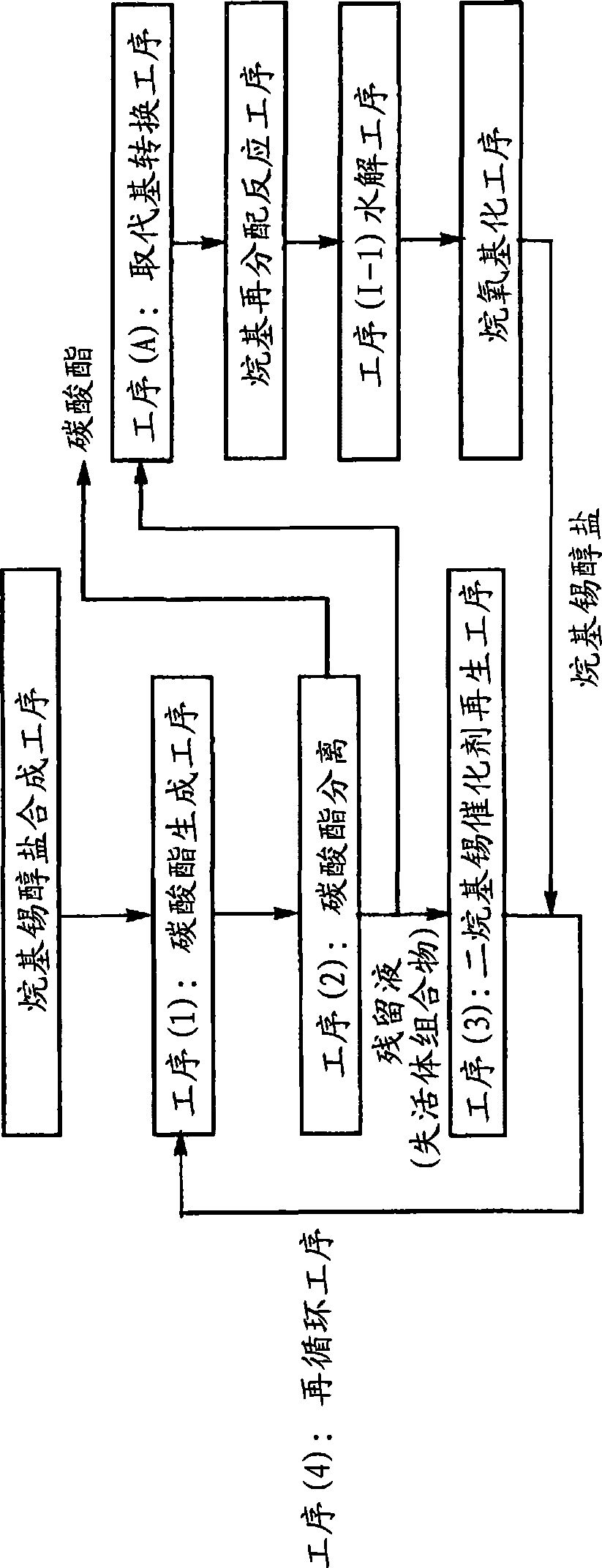
[0314] Step (1-1): Production of dialkyltin catalyst
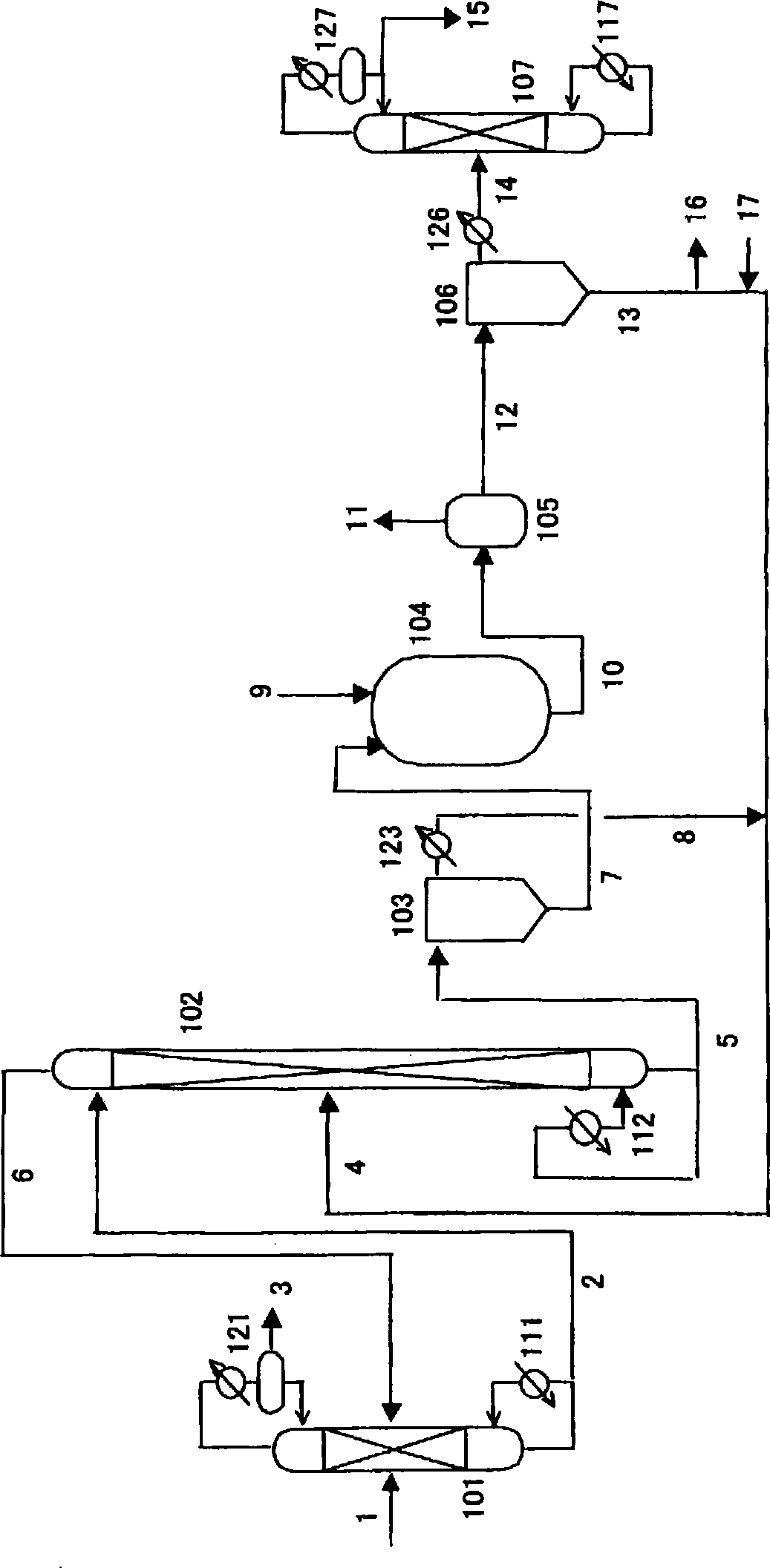
[0315] Add 627g (2.7mol) of dibutyltin oxide (manufactured by Sankyo Organic Synthesis Co., Ltd.) and 2000g (22.7mol) of 3-methyl-1-butanol (manufactured by Kuraray Corporation) into an eggplant-shaped flask with a capacity of 5000mL . The flask was installed in an oil bath with a temperature regulator (manufactured by Masuda Physical and Chemical Industry Co., Ltd., Japan, OBH-24), a vacuum pump (manufactured by Japan ULVAC Co., Ltd., G-50A) and a vacuum controller (manufactured by Okano Seisakusho, Japan). , VC-10S) evaporator (manufactured by Japan Shibata Corporation, R-144). The outlet of the purge valve of the evaporator is connected with the pipeline flowing nitrogen at normal pressure. Close the vent valve of the evaporator to depressurize the system, then slowly open the vent valve, flow nitrogen into the system, and return to normal pressure. The temperature of the oil bath was set to about 145°C, the flask wa...
Embodiment 2
[0331] Step (2-1): Substituent exchange reaction of deactivated form of dialkyltin catalyst
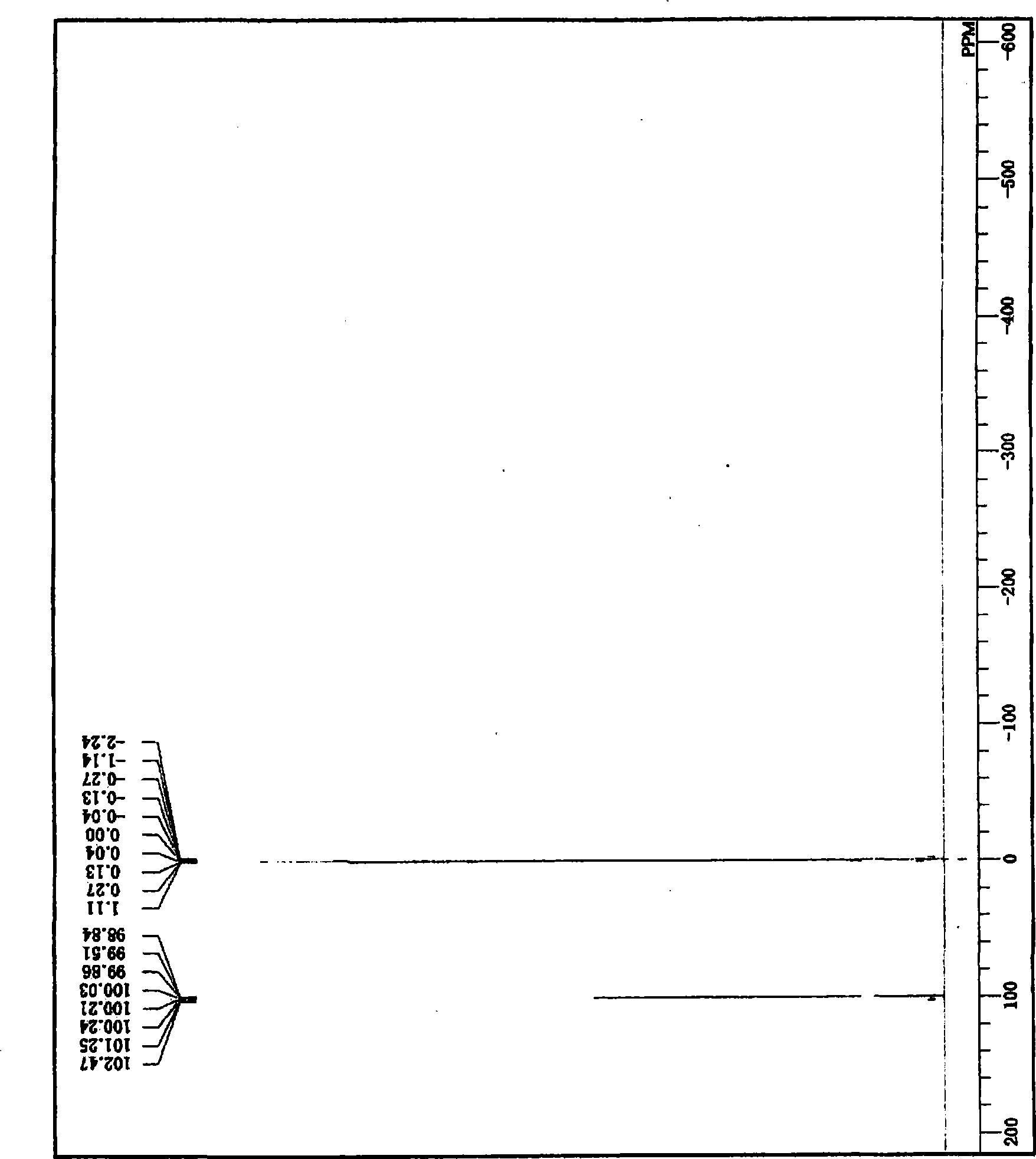
[0332] Under a nitrogen atmosphere, 125 g of the inactivated body composition obtained in the same manner as in the procedure (1-2) of Example 1 was added to a 500 mL eggplant-shaped flask, and then 145.0 g of acetic anhydride was added, and stirred at 25° C. for 1 Hour. A fractionating head with a reflux cooler connected to the distillate receiver and a thermometer were installed on the flask, and after vacuum-nitrogen exchange was performed in the flask, the flask was immersed in an oil bath heated to 50°C. The pressure in the container was gradually reduced, and the remaining acetic anhydride and the like were distilled off to obtain 125.9 g of a residue in the flask. carry out on the residue 1 H and 119 Sn-NMR measurement, the residue is tri-n-butylacetoxytin and di-n-butyldiacetoxytin and has 119 In Sn-NMR, a mixture of organotin compounds with tin atoms showing two or more c...
Embodiment 3
[0338] Step (3-1): Substituent exchange reaction of deactivated form of dialkyltin catalyst
[0339] 130 g of the inactivated body composition obtained by the same method as in the step (1-2) of Example 1 and 100 g of toluene (manufactured by Wako Pure Chemical Industries, Ltd., special grade) were put into a 500 mL eggplant-shaped flask. A Dean Stark tube, a Dimroth cooler and a three-way stopcock were installed on the eggplant flask. Connect the three-way cock to a line flowing with nitrogen at normal pressure.
[0340] This flask was immersed in an oil bath previously heated to 140° C., toluene was refluxed, and hydrogen chloride gas was flowed into this flask at a flow rate of about 150 mL / min. While the toluene was refluxing, water was recovered into the Dean Stark tube, and the reaction was terminated after 16 hours of reaction. After cooling the flask to around room temperature, nitrogen gas was released into the flask for 3 hours. Toluene was distilled off from the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com