Hematology linearity control composition system and method of use
A compositional and linear technology, applied in scientific instruments, biological tests, reference solutions, etc., can solve problems such as time-consuming and measurement errors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Examples 1 and 2 provide exemplary procedures for preparing leukocyte analogs for use in the linear control compositions of the invention. In Example 1, the leukocyte analog was a monocyte analog when measured by impedance as described in US Patent No. 4,704,364 (Carver et al.). In Example 2, the leukocyte analog is a lymphocyte analog as measured by the VCS measurement as described in US Patent No. 5,320,964 (Young et al.). Example 3 provides an exemplary process for preparing platelet analogs for use in linear control compositions. Additionally, Example 5 provides an exemplary process for preparing stabilized red blood cells that can be used in linear control compositions.
[0044] It was found that linear control compositions of the present invention containing high concentrations of leukocyte analogs, stabilized red blood cells, and optionally platelet analogs as described above exhibited both open vial stability and closed vial stability, which was essentially Th...
Embodiment 6
[0058] Example 6 provides an exemplary process for preparing a linear control system. As indicated, after all individual line control compositions were prepared and packaged into control vials, the control vials were labeled to show their levels in the line control system. The assay parameters were then assigned to the linear control composition at each level. As used herein, the term "measurement parameter" includes measured values and target ranges. Typically, measured values are presented as a single quantity with a margin of error; and target ranges are presented as acceptable upper and lower limits. For example, the WBC assay parameter at level 8 for the linear control system shown in Table 2 may either be an assay value of 290 ± 21.6, or a target range with an upper limit of 311.60 and a lower limit of 268.4.
[0059] Standard procedures known in the art for assay value assignment of hematological controls were used for the linear control system. For each batch, t...
Embodiment 7
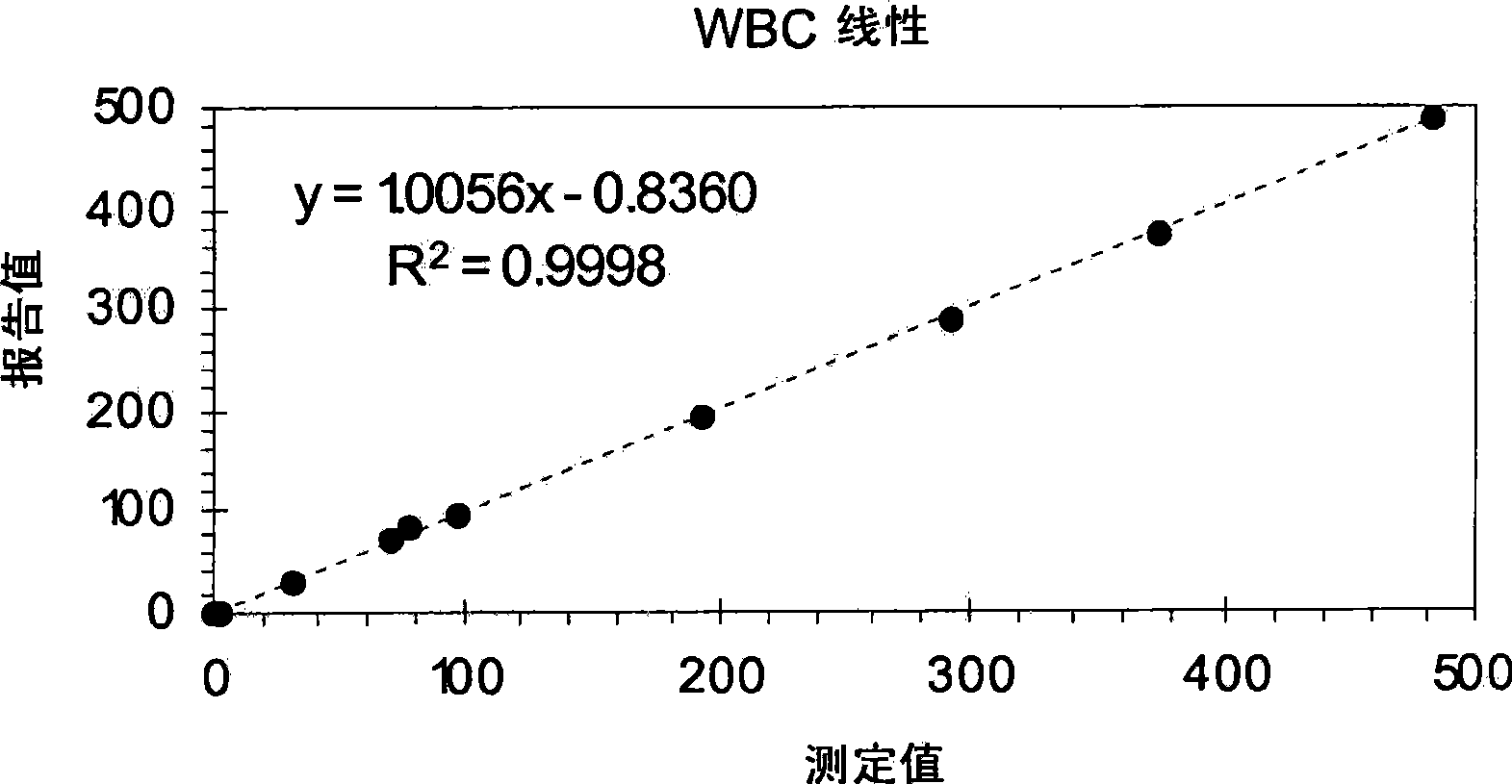
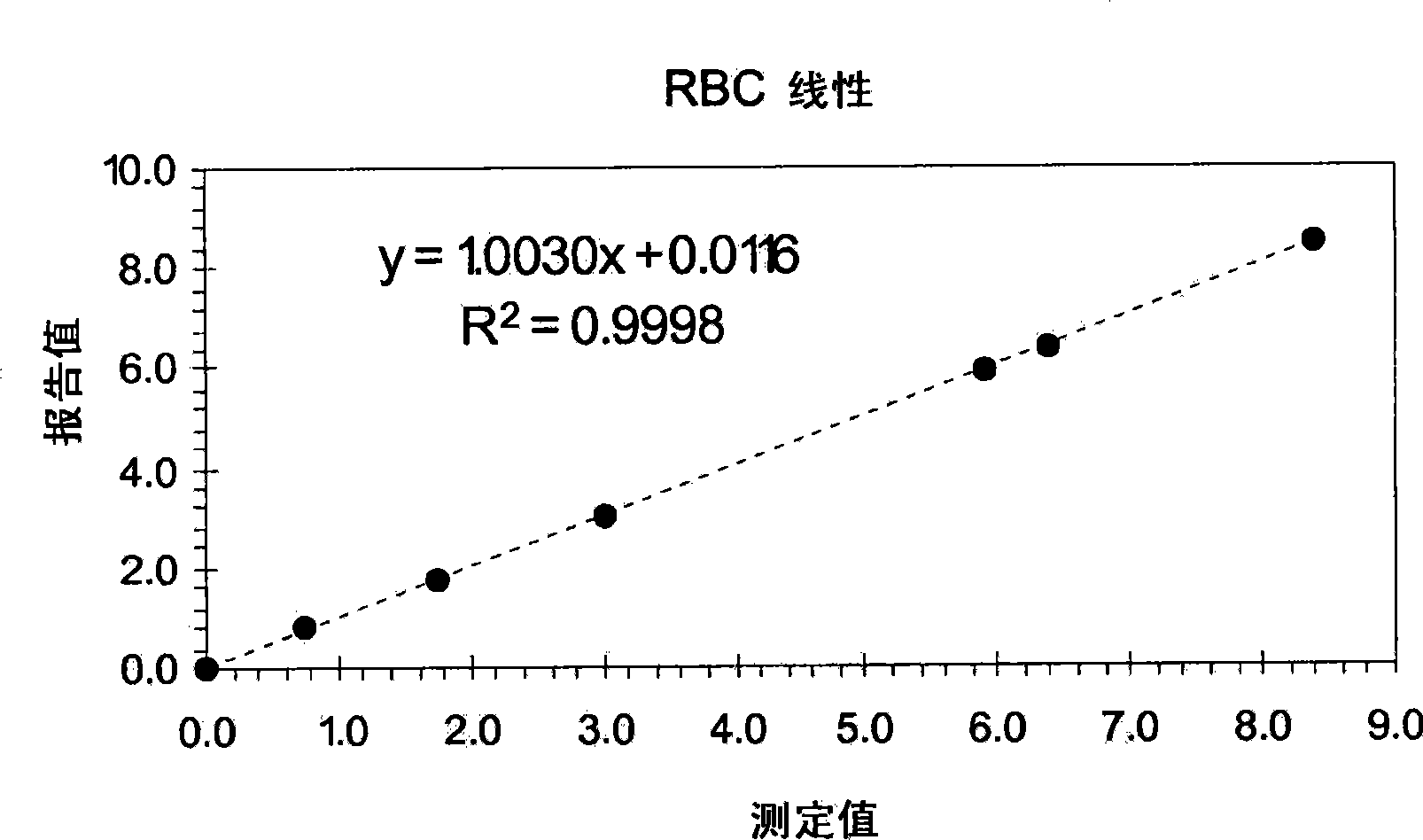
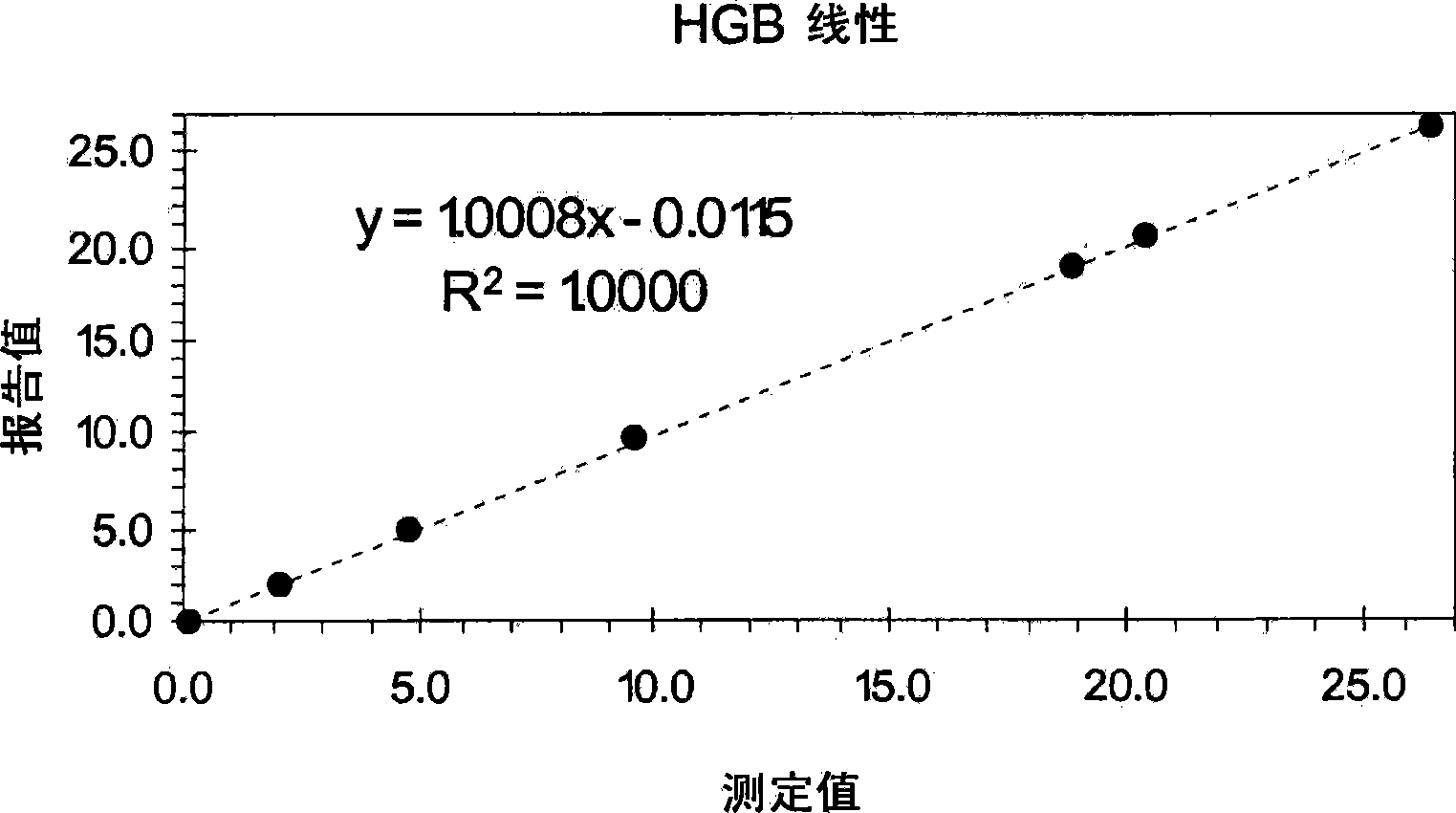
[0076] Example 7 provides an exemplary procedure for determining the linearity of white blood cell, red blood cell, and platelet counts, and hemoglobin measurements using a linear control system. As shown therein, the control vials were mixed by hand following a procedure that clinical laboratories have used for many years to process a baseline control containing cell particles equivalent to the concentration of cell particles in a normal blood sample. By using this procedure, the control vials were mixed gently by hand within tens of seconds without any vigorous mixing. Additionally, all levels of the control composition were used directly on the instrument without further dilution by the operator.
[0077] After obtaining the reported values for WBC, RBC, Hgb, and Plt for each level of the linear control system, and obtaining the average of the reported values for each of these four parameters, each of these reportable parameters The linearity of a measurement can be de...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com