Pseudomonad esterase and application in preparing optical pure mandel and derivative thereof
A technology for pseudomonas and derivatives, which is applied in the field of Pseudomonas esterase and its use for preparing optically pure mandelic acid and derivatives thereof, achieving high optical purity, good stability and wide industrial application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The cultivation of embodiment 1 bacterial strain CGMCC 2872
[0024] The strain CGMCC 2872 was inoculated into the seed liquid containing rich medium, and cultured at 30°C and 180rpm for 12 hours; the rich medium consisted of 15g of glycerol, 5g of peptone, 5g of yeast extract, 1g of NaCl, MgSO 4 0.2g, KH 2 PO 4 0.5g, K 2 HPO 4 0.5g, tap water 1000ml, adjust the pH value to 7.0. The seed solution was inoculated into the rich medium with an inoculation amount of 5% v / v, and cultured at 30° C. and 180 rpm for 18 hours. After the cultivation, centrifuge at 10,000rpm to obtain enzyme-containing bacteria.
Embodiment 2
[0025] Example 2 Different candidate strains for the hydrolysis of acetoxyphenylacetic acid
[0026] According to the method described in Example 1, the wet cells of different candidate strains were washed 3 times with physiological saline with a concentration of 0.85% w / v, and added to the substrate 2-acetoxy In a phosphate buffer solution with a pH of 7.0 and a phenylacetic acid concentration of 20 mmol / L, react at 30° C. and 180 rpm for 12 hours, and then add 0.1% v / v concentrated sulfuric acid to terminate the reaction. Add sodium chloride and ethyl acetate, shake and extract for 1 minute, centrifuge to take the supernatant, add anhydrous sodium sulfate and let stand overnight, and use liquid chromatography to detect the conversion rate of the reaction and the optical purity of the product.
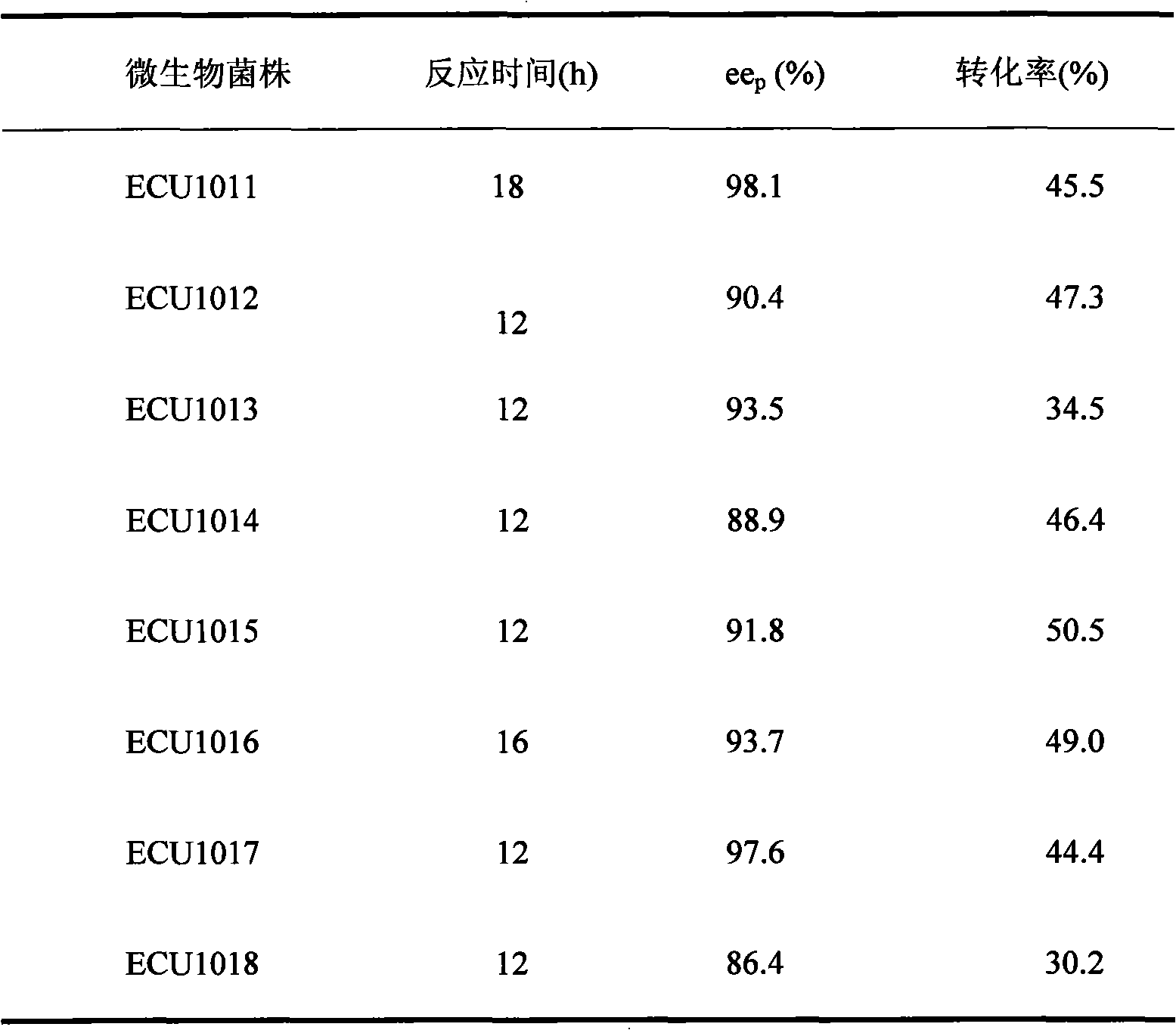
[0027] Table 1 Catalytic performance comparison of candidate strains
[0028]
[0029] The results in Table 1 show that the 8 strains screened from the soil all have high enantios...
Embodiment 3
[0030] The influence of embodiment 3 different substrate concentration on CGMCC 2872 reaction characteristics
[0031] According to the method described in Example 1, the wet cells of CGMCC 2872 were cultivated, washed 3 times with physiological saline with a concentration of 0.85% w / v, and added to the substrate 2-acetoxy Phenylacetic acid concentrations were 5, 10, 20, 40, 60 and 100mmol / L pH7.0 phosphate buffer solution, reacted for a certain period of time at 30°C and 180rpm, after confirming that the reaction was complete, add 0.1% v / v of concentrated sulfuric acid to terminate the reaction. Add sodium chloride and ethyl acetate, shake for 1 minute, centrifuge to take the supernatant, add anhydrous sodium sulfate and let it stand overnight, and use liquid chromatography to detect the conversion rate of the reaction and the optical purity of the product.
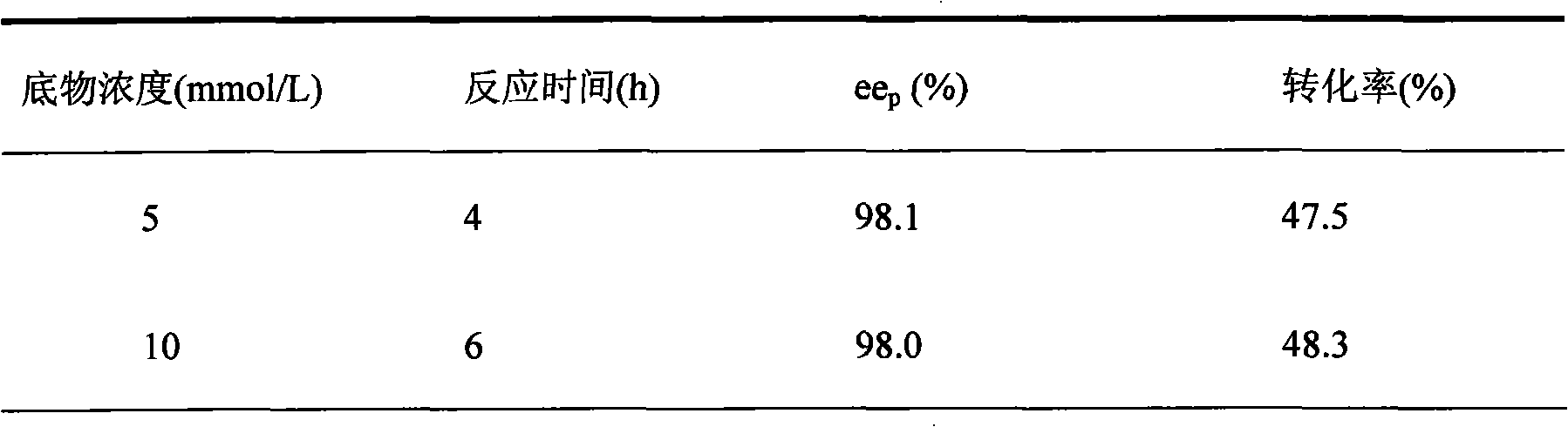
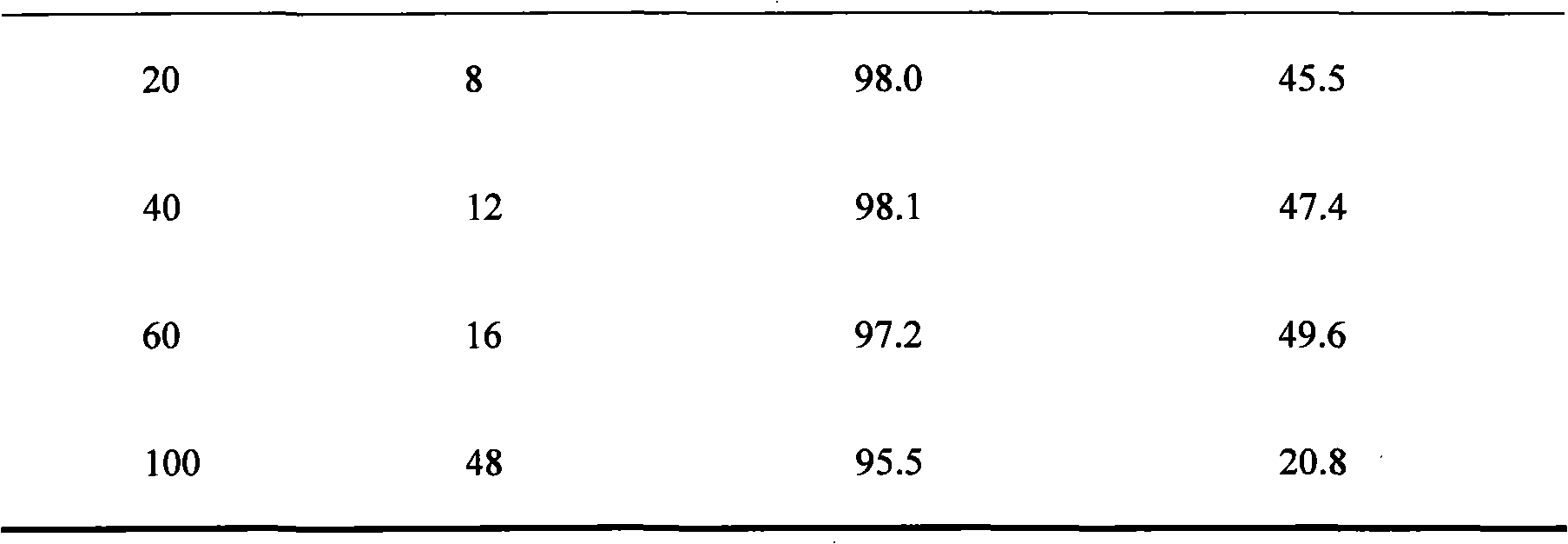
[0032] Table 2 Effects of different substrate concentrations on the reaction characteristics of CGMCC 2872
[0033]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com