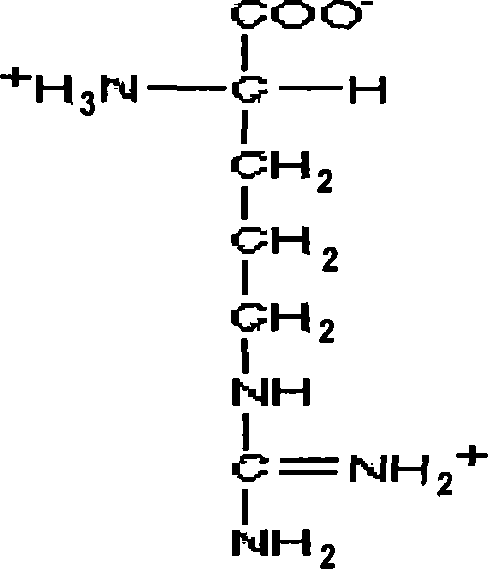Method and substance for keeping fibrinogen activity in thermal treatment
A technology for fibrinogen and protein activity, applied in the field of protein activity maintenance, can solve the problem of inactivation of non-lipid envelope and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
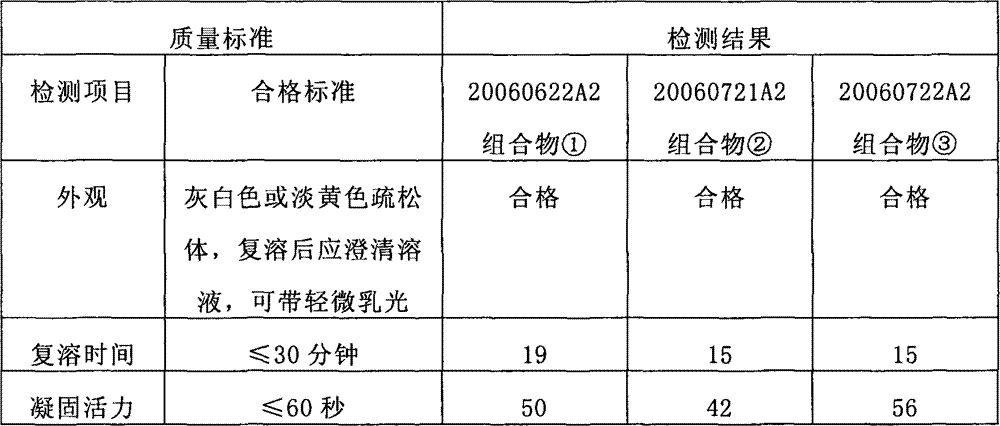
[0112] Preparation of fibrinogen composition①
[0113] Adjust the pH of human plasma to 5.0-9.0, add ethanol and centrifuge to obtain a precipitate rich in fibrinogen.
[0114] Dissolve the precipitate in sodium citrate-Tris buffer, pH 6.0-8.0, filter to remove insoluble particles, then add Tween 80 (Tween80) and tributyl phosphate (TNBP) to the solution to the final concentration of Tween80 TNBP is 1.0%, TNBP is 0.3%, the temperature of the solution is adjusted to 24-26° C., and it is incubated for at least 6 hours to inactivate the lipid-enveloped virus.
[0115] After S / D virus inactivation treatment, dilute with sodium citrate-Tris buffer solution, pH 6.0-8.0, cool down the solution, add ethanol and centrifuge to obtain a precipitate.
[0116] The precipitate was redissolved in sodium citrate-Tris buffer, the pH was 6.0-8.0, filtered, the solution was cooled down, and ethanol was added to centrifuge to obtain a purified precipitate.
[0117] One part of this precipitate ...
Embodiment 2
[0120] Preparation of fibrinogen composition②
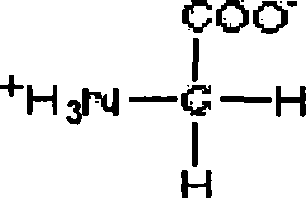
[0121] One part of this precipitate was dissolved in three parts at 25°C in sodium citrate-hydrochloric acid buffer, pH 6.0-8.0, and the resulting solution contained 2.0% fibrinogen, 5 mmol / L sodium citrate, L- Arginine hydrochloride 5g / L, glycine 5g / L.
Embodiment 3
[0123] Preparation of fibrinogen composition③
[0124] One part of this precipitate was dissolved in three parts at 25°C in sodium citrate-hydrochloric acid buffer solution, pH6.0-8.0, and the resulting solution contained 5.0% fibrinogen, sodium citrate 100mmol / L, L- Arginine hydrochloride 100g / L, glycine 100g / L.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com