Leptospira vaccine candidate outer membrane protein
A leptospira and outer membrane protein technology, applied in the fields of biotechnology and medicine, can solve the problems of narrow scope of action, insufficient immunity, and limited application of leptospira vaccines.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
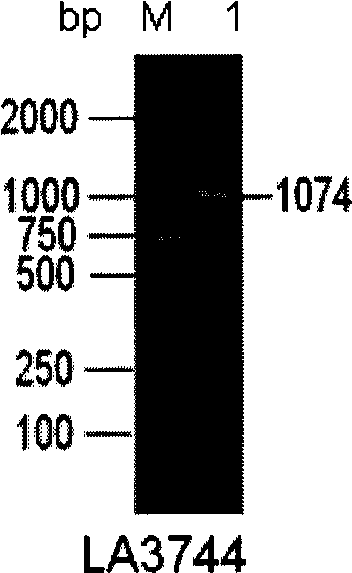
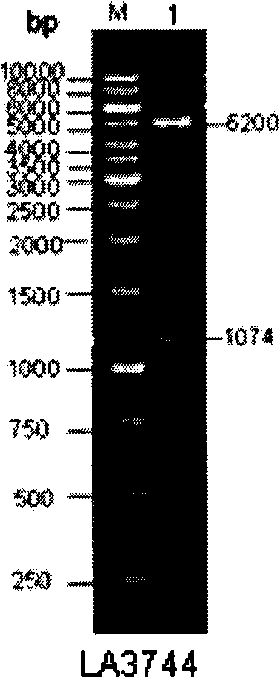
[0091] Example 1 Cloning, expression and identification of a new vaccine candidate gene LA3744
[0092] The LA3744 gene was cloned and expressed by conventional molecular biology methods, the expression vector was pET28b+, and the expression strain was E.coli BL21-3E. After screening positive clones for Kan resistance, they were added to LB liquid medium and amplified overnight at 37°C and 250rpm. Transfer to LB medium at an inoculum size of 1:100, culture at 37°C for 2.5hrs until the OD is about 0.6, add IPTG to a final concentration of 0.5mM, induce protein expression at 30°C 250rpm for 8hrs. Ultrasonic crush the E.coli BL21-3E cells that have expressed the protein, and collect the precipitate. According to the method provided by Qiagen, the components containing the recombinant protein were collected by Ni-NTA affinity chromatography, and subjected to dialysis and renaturation. The results of PCR amplification products, enzyme digestion identification of recombinant pla...
Embodiment 2
[0094] Example 2 Immunogenicity detection of recombinant proteins
[0095] 400 μg of recombinant protein plus an equal volume of complete Freund’s adjuvant (Sigma Company) was used to immunize 3 kg of New Zealand rabbits. PBS + adjuvant was used as a negative control group. After the initial immunization, 200 μg of recombinant protein plus an equal volume of incomplete Freund’s adjuvant was used to boost once every 15 days , blood was collected on the 10th day after two times of strengthening, and the potency was measured by ELISA. The antibody titer produced by recombinant protein PL40 is greater than 1:16000. , showing good immunogenicity. The immunoreactivity of the recombinant protein was detected by Western-blot, which showed that the prepared polyclonal antibody could effectively bind to the corresponding recombinant protein.
Embodiment 3
[0096] Example 3 Conservative Detection of LA3744 and Its Encoded Protein in Leptospira Endemic Strains in China
[0097]Conservation detection at the nucleic acid level: Using 15 Chinese strains and the total DNA of the double helix as templates, primers were designed for the conserved regions for PCR amplification. Specific amplified bands were produced in 14 strains, while Weissner No specific bands were produced in Leptospira clear water type L 105 strain and non-pathogenic hyperbolic Leptospira ( Figure 5 ).
[0098] Conservation detection at the protein level: PL40 antiserum was used to detect the expression of representative strains of 15 serogroups prevalent in China by Western blot. Corresponding hybridization bands can be detected in 14 strains, and the molecular weight is consistent with the theoretical value, indicating that the protein is expressed, but it is not detected in the L105 strain of Leptospira wesmanii Shimizu type and the non-pathogenic Leptospira...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com