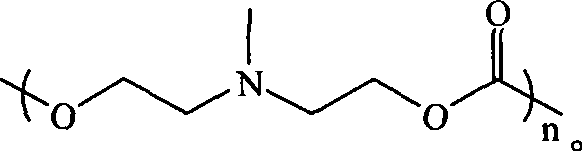
Biodegradable water-soluble polycarbonate and method for preparing same
A polycarbonate, water-soluble technology, applied in the field of synthetic chemistry, can solve problems such as unfavorable large-scale production and practical application, cumbersome and other problems, and achieve the effects of high biocompatibility, high material safety, and simple preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Get 0.5mol N-methyldiethanolamine, 0.6mol diethyl carbonate and 0.003mol K 2 CO 3 , added to a 150ml single-necked flask, and refluxed at 100°C for 2 hours. After fractional distillation at 130°C for 5 hours to remove by-products, a dark brown viscous liquid was obtained. The dark brown viscous liquid was washed with 50ml CH 2 Cl 2 Extraction, wash with 30ml saturated brine each time, wash twice, collect the organic phase, then anhydrous Na 2 SO 4 Dry well. Filter the dried product, remove the solvent, and crack under vacuum at 230°C to obtain a yellow solid-liquid mixture, which is then recrystallized in ethyl acetate to obtain white needle-like crystals with a yield of 20-30%. Through mass spectrometry (MS), infrared (IR), and nuclear magnetic (NMR) characterization analysis, it was confirmed that the white crystal was 6,14-dimethyl-1,3,9,11-tetraoxo-6,14-diazo-cyclo Hexadexane-2,10-dione (ie (ADMC) 2 ). MS: M + =291.IR:v=1746cm -1 (C=O). 1 HNMR (CDCl 3 )...
Embodiment 2
[0016] Get 0.5mol N-methyldiethanolamine, 0.55mol diethyl carbonate and 0.002mol K 2 CO 3 Add it to a 150ml single-necked flask, and reflux at 80°C for 4 hours. After fractional distillation at 120°C for 10 hours to remove by-products, a dark brown viscous liquid was obtained. The dark brown viscous liquid was extracted with 50ml of chloroform, washed with 30ml of saturated brine each time, washed twice, the organic phase was collected, and then anhydrous Na 2 SO 4 Dry well. The dried product was filtered, the solvent was removed, and it was cracked in vacuum at 250°C to obtain a yellow solid-liquid mixture, which was then recrystallized in tetrahydrofuran to obtain white needle-like crystals. Carry out characterization analysis by the method identical with embodiment 1, confirm that this white needle-shaped crystal is (ADMC) 2 .
[0017] Take 50mg of dry monomer (ADMC) 2 , 2.5mg Novozym435 and 0.01ml toluene were placed in a 5ml small test tube, sealed with paraffin, a...
Embodiment 3
[0020] Get dry monomer (ADMC) in 50mg embodiment 1 2 , 10 mg Novozym435 and 0.3 ml toluene were placed in a 5 ml small test tube, sealed with paraffin, and reacted at 60° C. for 24 hours. After the reaction, the product was washed with 3ml CH 2 Cl 2 Dissolve, remove most of the solvent by filtration, and then use ether to make it completely precipitate, collect the precipitate, and dry to obtain the final product. The product was proved to be P(ADMC) by infrared and nuclear magnetic analysis.
[0021] The number average molecular weight of the obtained polymer was Mn=1958 g / mol. Polymer Dp = 13.5.
[0022] Embodiment of the invention (ADMC) 2 The preparation method is to use saturated brine to wash the extract, and other washing liquids can also be used, as long as the extract can be washed clean and does not react with the extract; after collecting the organic phase, use anhydrous Na 2 SO 4 For drying, other desiccants can also be used, as long as they can remove the w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com