Design and synthesis of novel tubulin polymerization inhibitors: benzoylphenylurea (bpu) sulfur analogs
A technology of compounds and hydrates, applied in the field of design and synthesis of novel tubulin polymerization inhibitors benzoylphenylurea (BPU) sulfur analogues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0258] Embodiment 1: the general method of synthesizing BPU sulfur analog
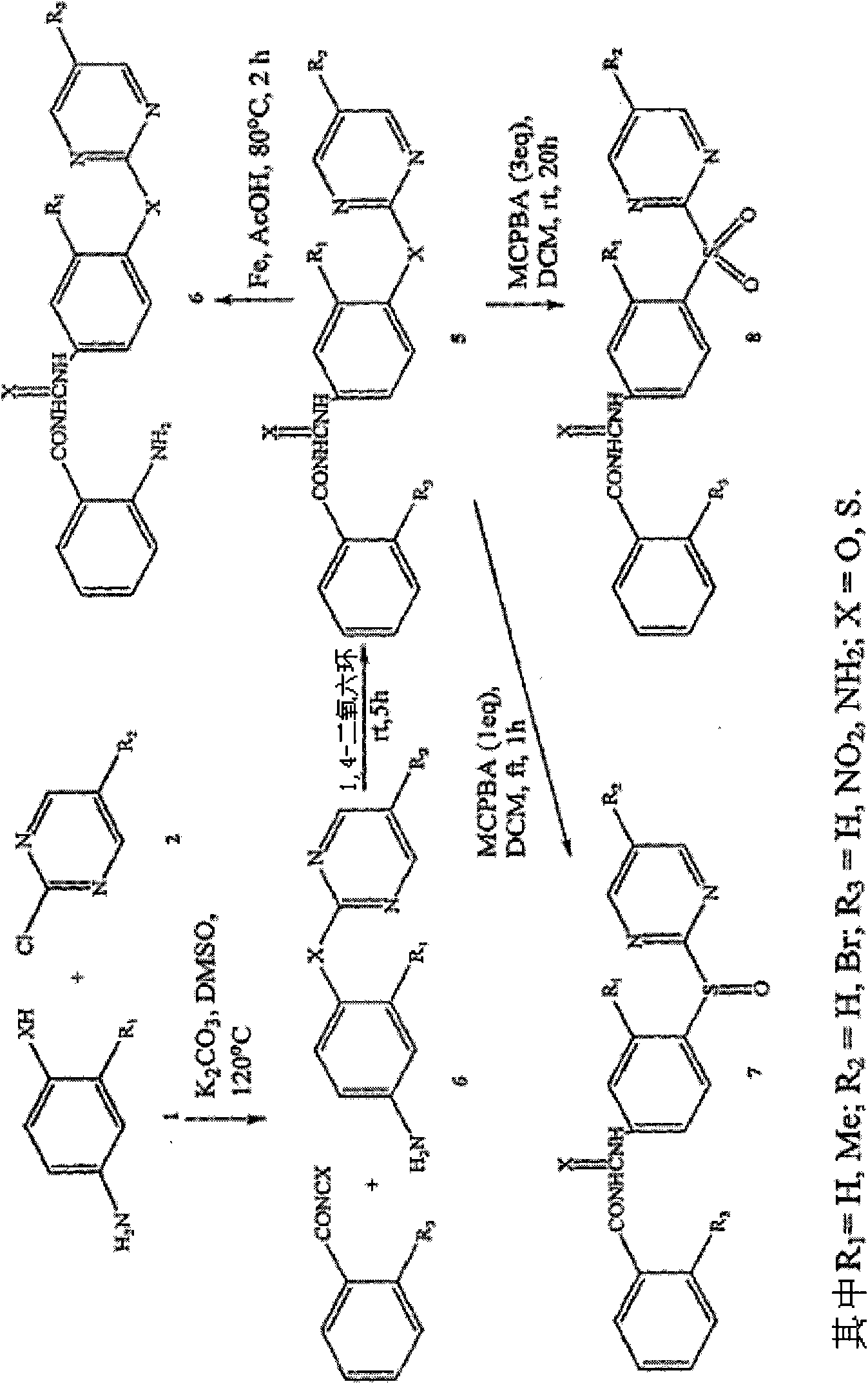
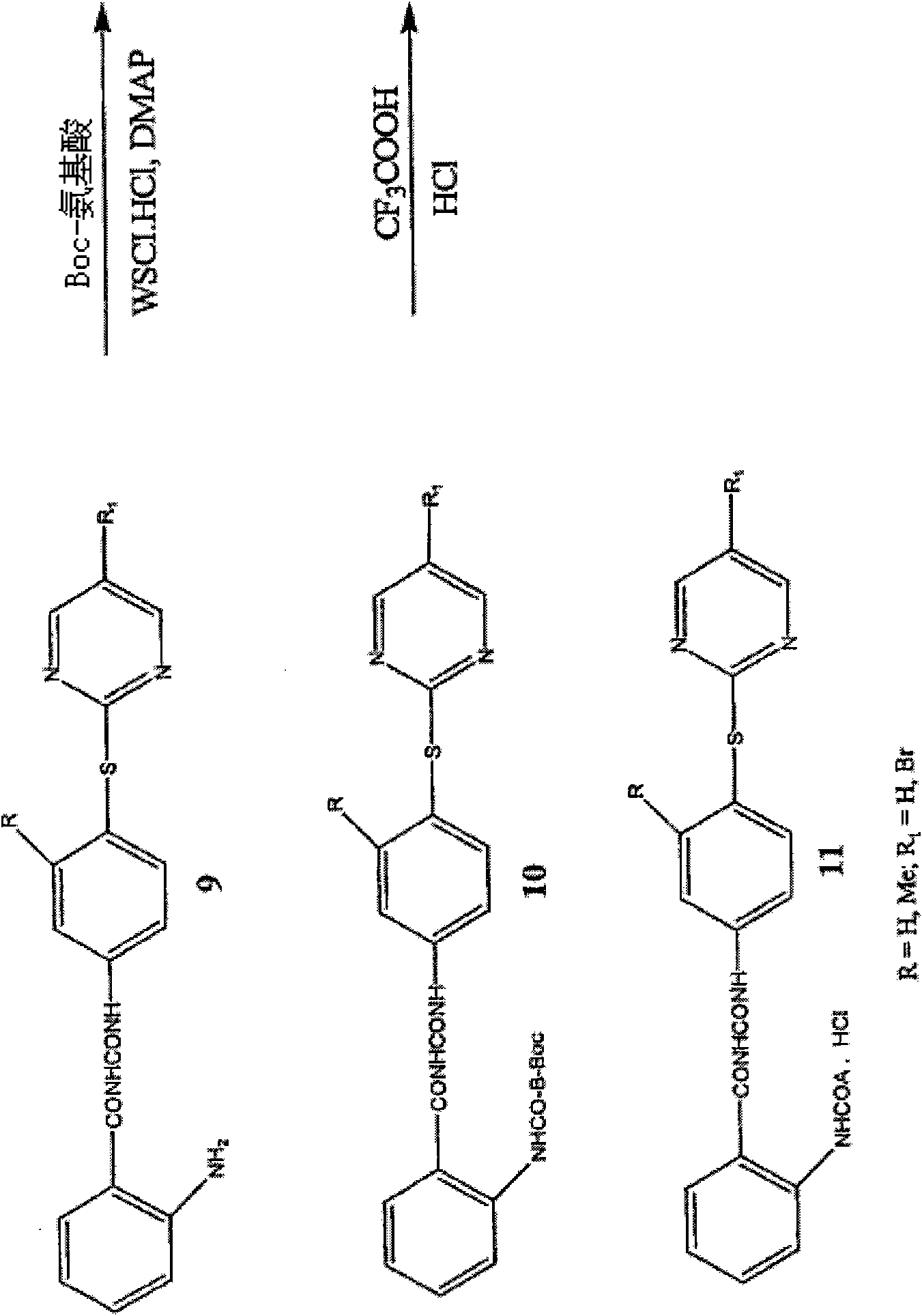
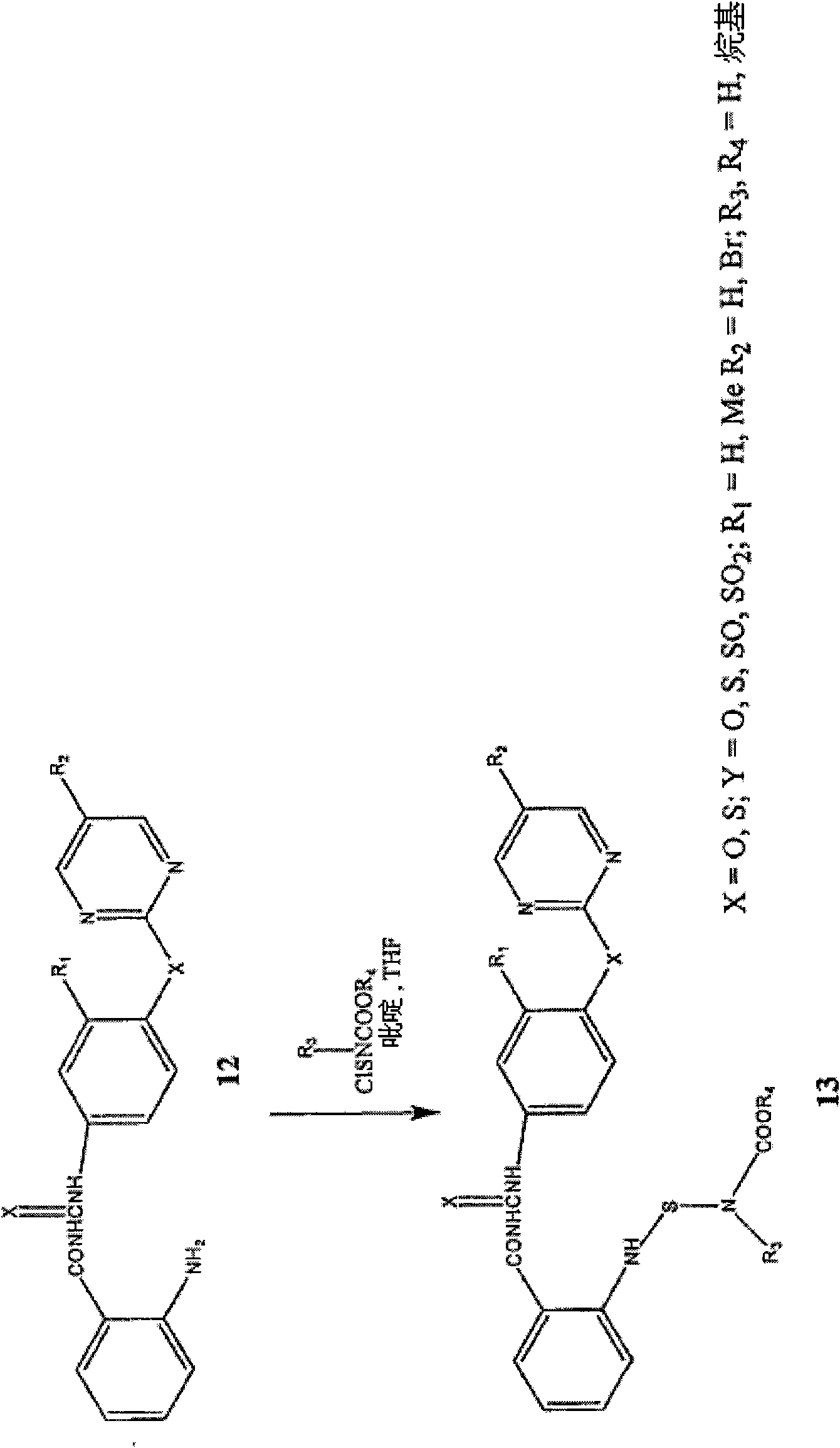
[0259] The new analogs were synthesized in good yields by coupling the corresponding benzoyl isocyanate or benzoyl isothiocyanate with aniline derivatives. Sulfur analogs of aniline intermediates were synthesized by reacting substituted aminothiophenols with aryl halides. Sulfoxide or sulfone derivatives are prepared by oxidation of sulfide ( figure 1 ). All compounds were characterized by NMR and LC-MS studies.
[0260] Unless otherwise stated, reactions were performed in oven-dried round bottom flasks under an atmosphere of ultra-high purity (UHP) argon. All reactive liquid reagents were transferred by syringe or cannula and added to flasks through rubber septa. THF was freshly distilled from the benzophenone carbonyl radical immediately before use. All other solvents and reagents were used as received unless otherwise stated. n-BuLi was obtained commercially and titrated with N-pivaloyl-O-t...
Embodiment 2
[0281] Example 2: Cytotoxic evaluation of BPU sulfur analogues
[0282] The cytotoxicity of eight of these analogues 6d, 6h, 6n, 6g, 7b, 7d, 8g and 8h in seven pancreatic cell lines ASPC1, Panc1, Panc203, Panc430, Pane1005, MiaPaca2 and HS766T was determined using the MTT assay. Trypsinize the cells to 5 x 10 3 Cells / well were seeded in 96-well plates and cells were allowed to grow in 10% FBS for 24 hours before treatment with exponentially increasing drug concentrations. After a 96 hour treatment period, 20 μl of MTT solution (5 mg / ml in PBS) was added to each well and the plates were incubated at 37° C. for 3 hours. The medium in each well was then replaced with 100 μl of DMSO. The plate was shaken and the optical density was measured at 570 nm using a multiwell plate reader (Bio-Rad, Model 550, Bio-Rad Inc., Hercules, CA). Each experiment was performed in triplicate for each drug concentration and performed independently at least 3 times. IC 50 Values are defined a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com