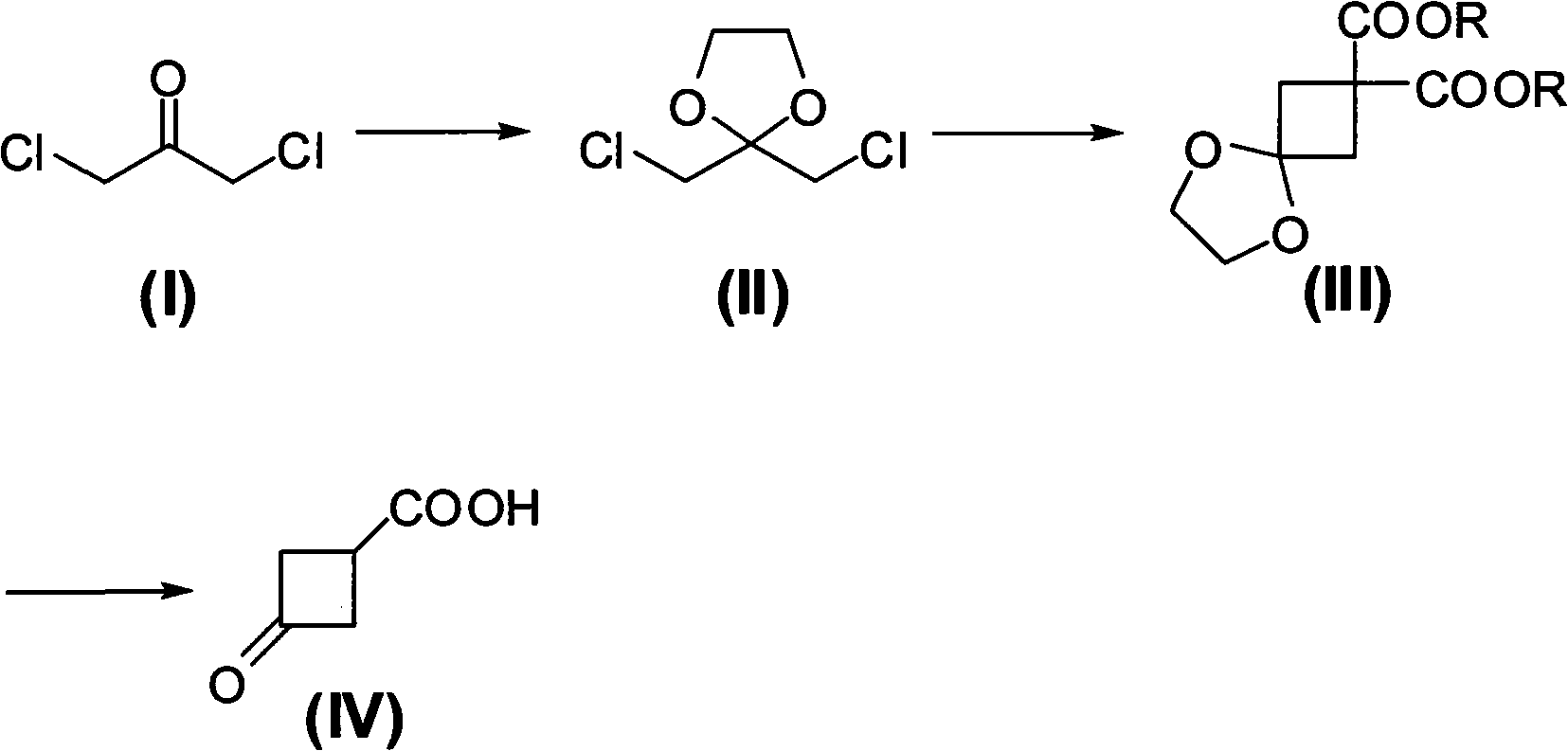
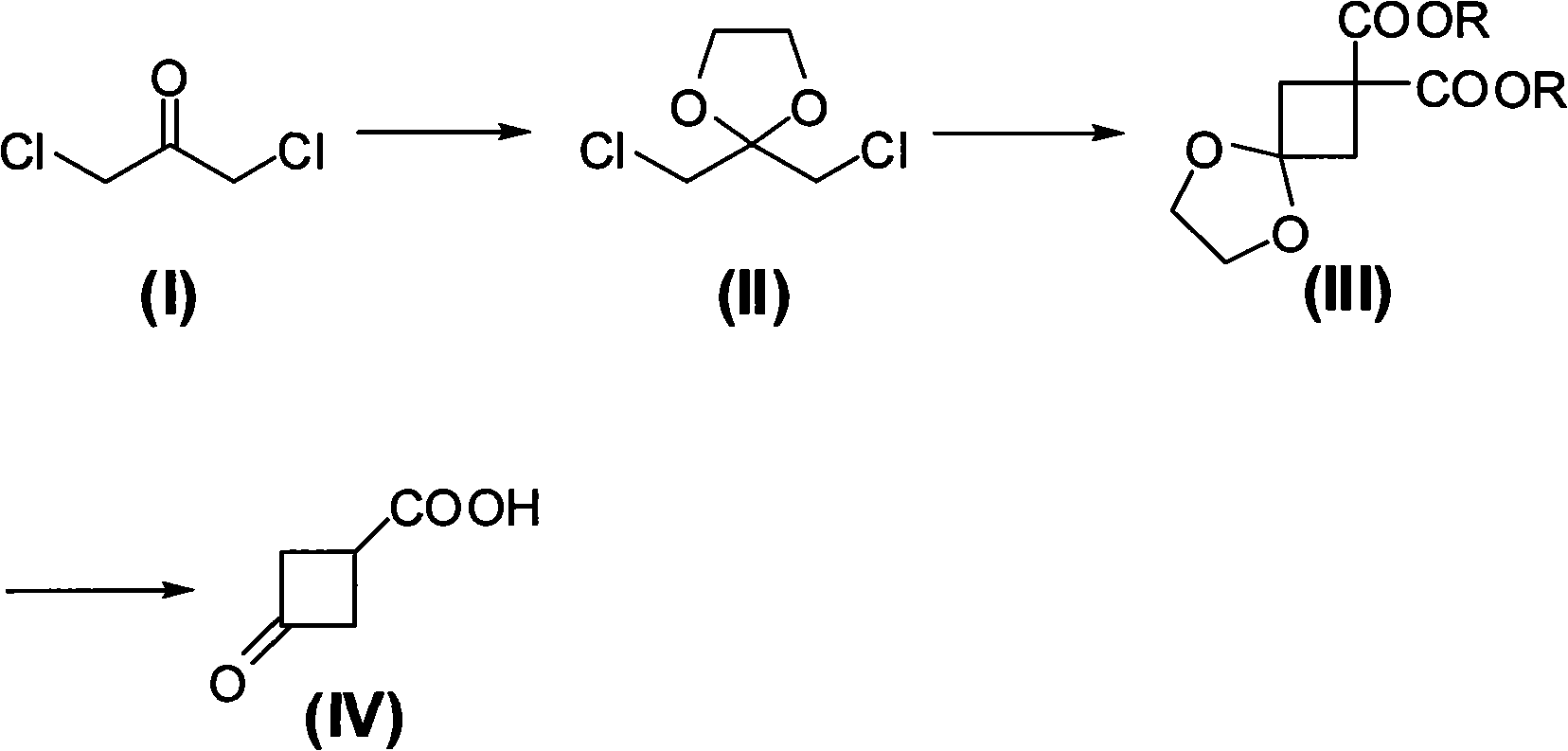
Preparing method of 3-oxo-1-cyclobutane-carboxylic acid
A technology of cyclobutane carboxylic acid and oxo generation, which is applied in the field of preparation of pharmaceutical and chemical intermediates, can solve the problems of unsatisfactory product quality, expensive osmium tetroxide, and difficulty in large-scale production, and achieve easy control and control of production costs , cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] A. Preparation of 2,2-dichloromethyl-1,3-dioxolane
[0023] Add 1,3-dichloroacetone (12.7g, 0.1mol), toluene (100ml), ethylene glycol (7.5g, 0.12mol) and p-Toluenesulfonic acid (0.3g), heated up to 100°C and stirred to react, then refluxed at this temperature to divide water until the distillate was clear liquid, about 6 hours, then cooled to room temperature, filtered to remove insoluble matter, the filtrate Raise the temperature to 120°C to distill off toluene, and the obtained residue is crude 2,2-dichloromethyl-1,3-dioxolane, and collect the 88-92°C / 10mmHg fraction by distillation under reduced pressure, namely 2,2-dichloromethyl-1,3-dioxolane Chloromethyl-1,3-dioxolane (14.7 g), yield 86.0%.
[0024] B. Preparation of diisopropyl 5,8-dioxaspiro[3,4]octane-2,2-dicarboxylate
[0025] In a 500ml three-neck flask equipped with a reflux condenser, a thermometer and a magnetic stirring device, add DMF (N,N-dimethylformamide, 80ml), and add sodium hydride (5.5g, 0.24mol...
Embodiment 2
[0029] Other steps are identical with embodiment 1, just the 2 of A step, the preparation method of 2-dichloromethyl-1,3-dioxolane is as follows:
[0030] Add 1,3-dichloroacetone (12.7g, 0.1mol), toluene (35ml), ethylene glycol (6.8g, 0.11mol) and p-Toluenesulfonic acid (0.13g), heated up to 100°C and stirred to react, then refluxed at this temperature to divide water until the distillate was clear liquid, about 6 hours, then cooled to room temperature, filtered to remove insoluble matter, the filtrate Raise the temperature to 120°C to distill off toluene, and the obtained residue is crude 2,2-dichloromethyl-1,3-dioxolane, and collect the 88-92°C / 10mmHg fraction by distillation under reduced pressure, namely 2,2-dichloromethyl-1,3-dioxolane Chloromethyl-1,3-dioxolane (13.8 g), yield 80.7%.
Embodiment 3
[0032] Other steps are identical with embodiment 1, just the 2 of A step, the preparation method of 2-dichloromethyl-1,3-dioxolane is as follows:
[0033] Add 1,3-dichloroacetone (12.7g, 0.1mol), toluene (100ml), ethylene glycol (9.9g, 0.16mol) and p-Toluenesulfonic acid (0.3g), heated up to 100°C and stirred to react, then refluxed at this temperature to divide water until the distillate was clear liquid, about 6 hours, then cooled to room temperature, filtered to remove insoluble matter, the filtrate Raise the temperature to 120°C to distill off toluene, and the obtained residue is crude 2,2-dichloromethyl-1,3-dioxolane, and collect the 88-92°C / 10mmHg fraction by distillation under reduced pressure, namely 2,2-dichloromethyl-1,3-dioxolane Chloromethyl-1,3-dioxolane (14.9 g), yield 87.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com