Preparation method of difenoconazole technical material
A difenoconazole and production method technology, applied in the field of triazole fungicides, can solve the problems of easy recovery, high isomer content, low bromide conversion rate, etc., and achieve improved yield, high conversion rate, Effect of reducing isomer content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
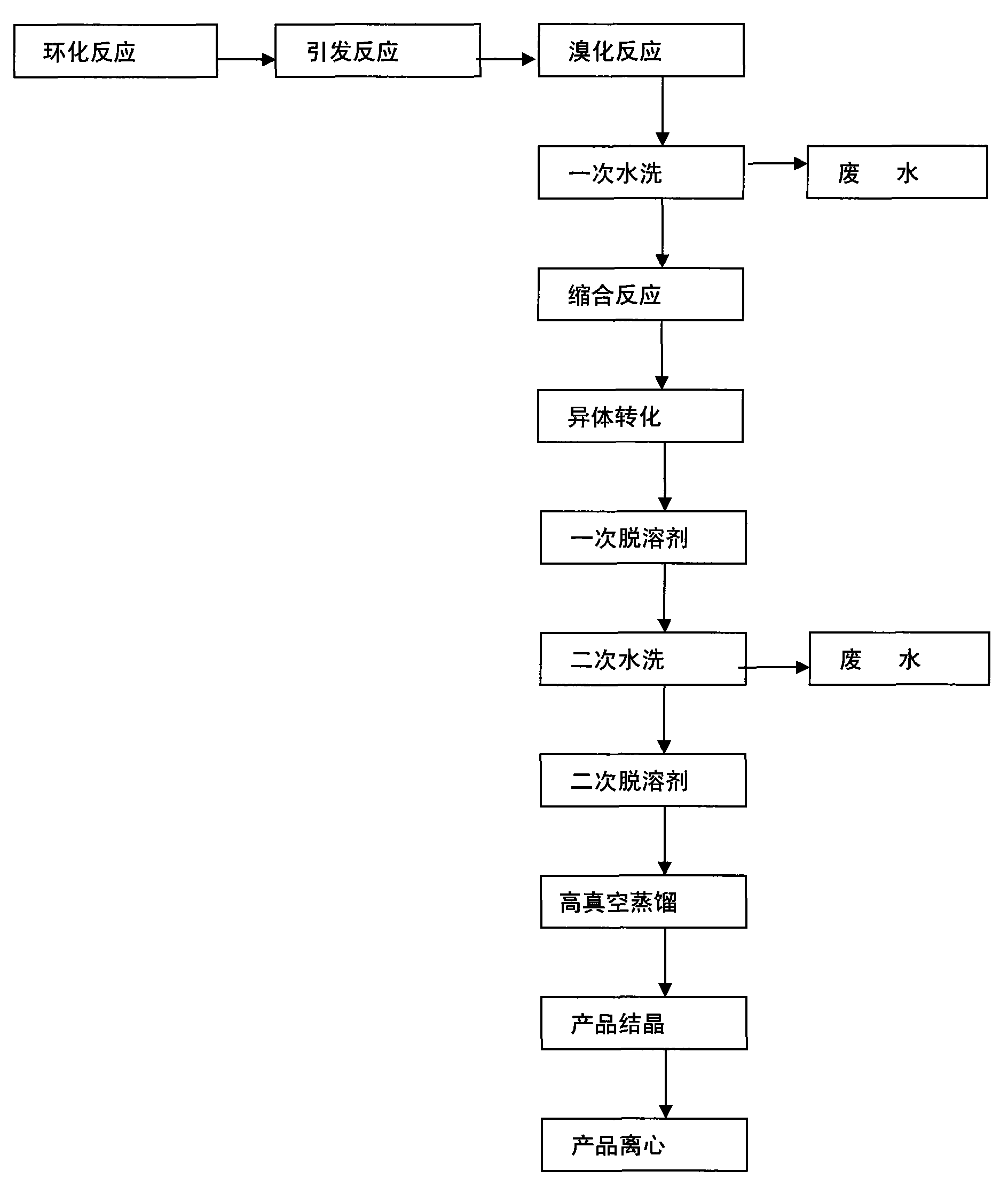
Image
Examples
Embodiment 1
[0012] In the 3000L enamel reaction kettle, add 4-(4-chlorophenoxy group)-2-chloroacetophenone 500KG of content 98%wt, under solvent hexanaphthene 2000L and catalyzer p-toluenesulfonic acid condition and 1 , 2-propanediol 160 ~ 200KG reflux reaction, continuous separation of water, to obtain a cyclohexane solution containing cyclized products, after making phenyl ether ketone ≤ 1.0 (liquid chromatography, L = 150mm, T = 40 ° C, wavelength 220) , lower the temperature to 20-25°C, and let stand to remove excess 1,2-propanediol. Then add 3% to 10% of the total amount of bromine required into the reaction kettle, and keep it warm for about 10 to 30 minutes at 20 to 25°C. When white smoke is emitted from the kettle, it proves that the bromination initiation is successful. Add the remaining 90%-97% bromine dropwise at 20-25°C, and the time is controlled within 2-3 hours. After dripping, keep warm for 1 hour, add water to wash until neutral, and evaporate cyclohexane under reduced p...
Embodiment 2
[0019] Under the conditions of Example 1, add 1,2,4-triazole 150KG (92%wt) and potassium hydroxide 142KG (92%wt) in the enamel reaction kettle, under the condition of toluene as solvent, reflux at 110°C water to obtain potassium 1,2,4-triazole, evaporate toluene, add 1800L of N-methylpyrrolidone, add the bromide prepared in Example 1, and react at 155°C to 160°C for about 20 hours to obtain diphenoxymethylcycline For crude azoles, evaporate pyrrolidone, add benzyl bromide accounting for 0.5% of the mass of the crude product, and keep warm for 1 to 3 hours at 200°C to 300°C for isomer conversion, then enter the high vacuum distillation process for distillation, and then go through the crystallization process (See Example 3 for details), to obtain difenoconazole.
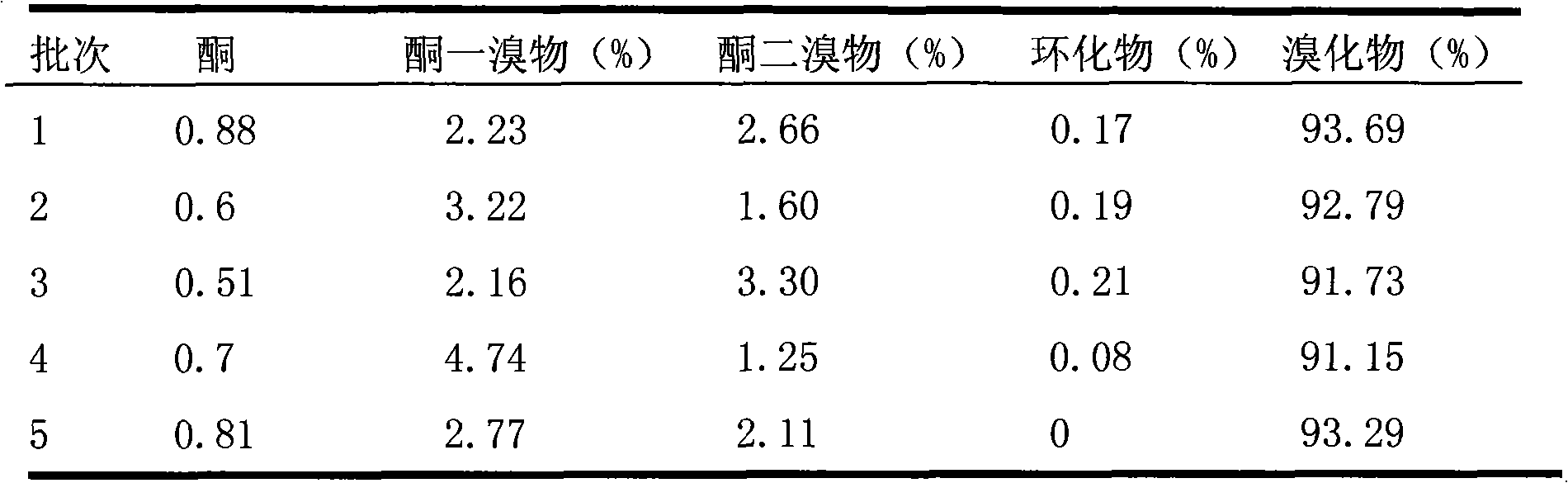
[0020] Table 3 Without the use of isomer conversion agent
[0021]
[0022]
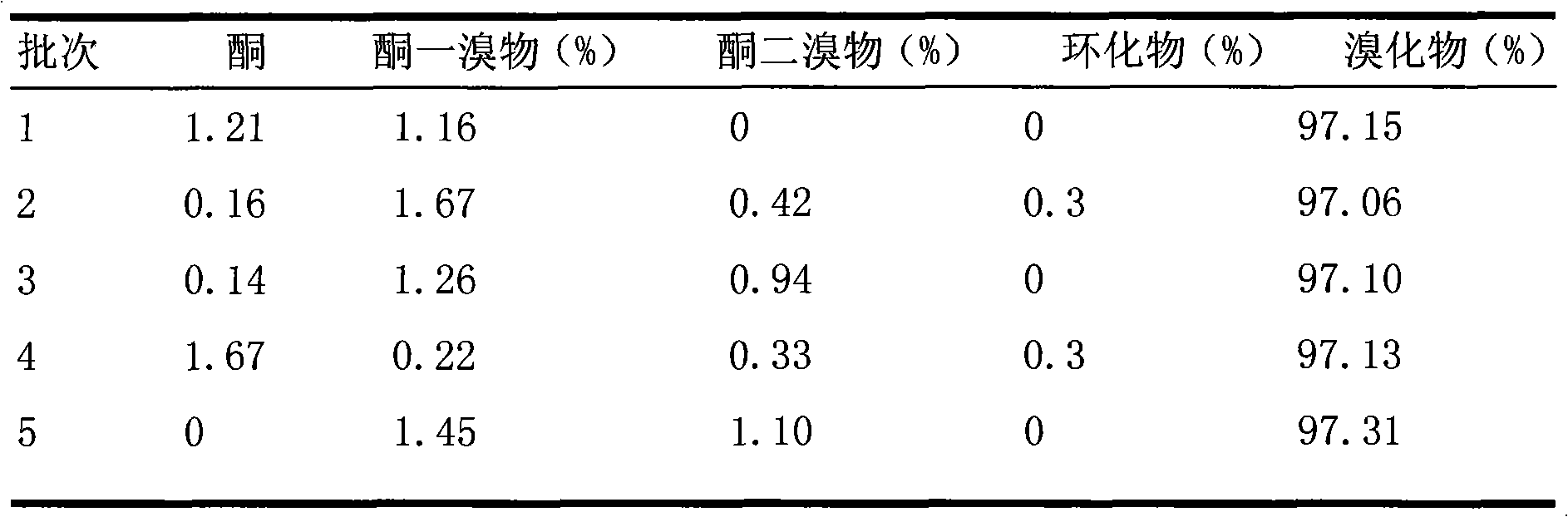
[0023] Table 4 using isomer conversion agent
[0024]
[0025] The results show that: the quality and yield of difenoconazole ar...
Embodiment 3
[0027] Under the conditions of Example 1 and Example 2, difenoconazole oil obtained by high-vacuum distillation was added to an enamel reaction kettle, and a single solvent methanol was added, heated to 60°C to dissolve it, kept for one hour, and then cooled to 10 ℃, add a small amount of crystallization aid polyacrylamide, the crystallization aid accounts for 0.05-10% of the mass of methanol, keep at 10°C for 10 hours, at 5°C for 10 hours, at 0°C for 10 hours, at -5°C for 8 hours Hours, after centrifugation to obtain a wet product, put the wet product into the enamel reaction kettle, add 0.5 times of fresh methanol, then cool down to -5 ° C for 5 hours, and then centrifuge to obtain a wet product.
[0028] The feasibility of long-term application of solvents is verified theoretically and practically. Prior to the present invention, binary solvents were used as alcohols and ethers with a fixed volume ratio. Due to their different boiling points, ethers are volatile substances...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com