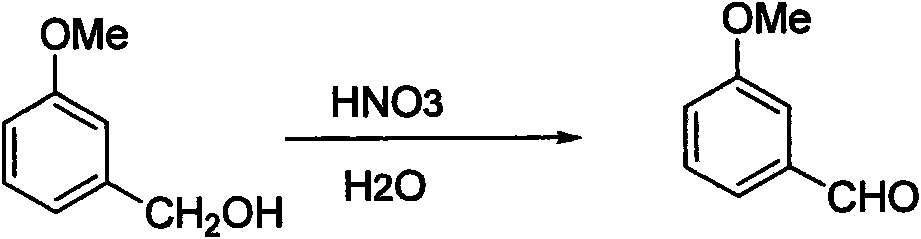
Method for synthesizing 3-methoxybenzaldehyde
A technology of methoxybenzaldehyde and methoxybenzyl alcohol is applied in the synthesis field of 3-methoxybenzaldehyde, can solve the problems of high cost and the like, and achieves the effects of easy operation, environmental friendliness, and cheap and readily available raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] The molar ratio of nitric acid and m-methoxybenzyl alcohol is 1.5, and the mass ratio of water and m-methoxybenzyl alcohol is 1;
[0016] Lower the two-necked bottle below 5 degrees, then add m-methoxybenzyl alcohol, start stirring, then add the nitric acid solution prepared in advance, after the addition, the system returns to room temperature, TLC and liquid phase monitor the reaction. The system was neutralized by adding sodium hydroxide solution, extracted with dichloromethane, washed with water and saturated brine, and dried over anhydrous magnesium sulfate. The product was obtained by precipitation under reduced pressure. Yield: 94%. 1 HNMR (300M, CDCl 3 ) δ (ppm) 9.87 (s, 1H, CHO), 3.73 (s, 3H, CH 3 ), 7.05(d, 1H, ArH), 7.32-7.37(m, 3H, ArH).
Embodiment 2
[0018] The molar ratio of nitric acid to m-methoxybenzyl alcohol is 1, and the mass ratio of water to m-methoxybenzyl alcohol is 0.8 to 1.5; the steps are the same as in Example 1, and the yield is 71%.
Embodiment 3
[0020] The molar ratio of nitric acid to m-methoxybenzyl alcohol is 2, and the mass ratio of water to m-methoxybenzyl alcohol is 1.5; the steps are the same as in Example 1, and the yield is 73%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com