Polyunsaturated fatty acid production in heterologous organisms using pufa polyketide synthase systems
A technology of organisms and enzyme synthesis, applied in the direction of biochemical equipment and methods, microbial measurement/inspection, etc., can solve problems such as inaccurate mechanism definition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
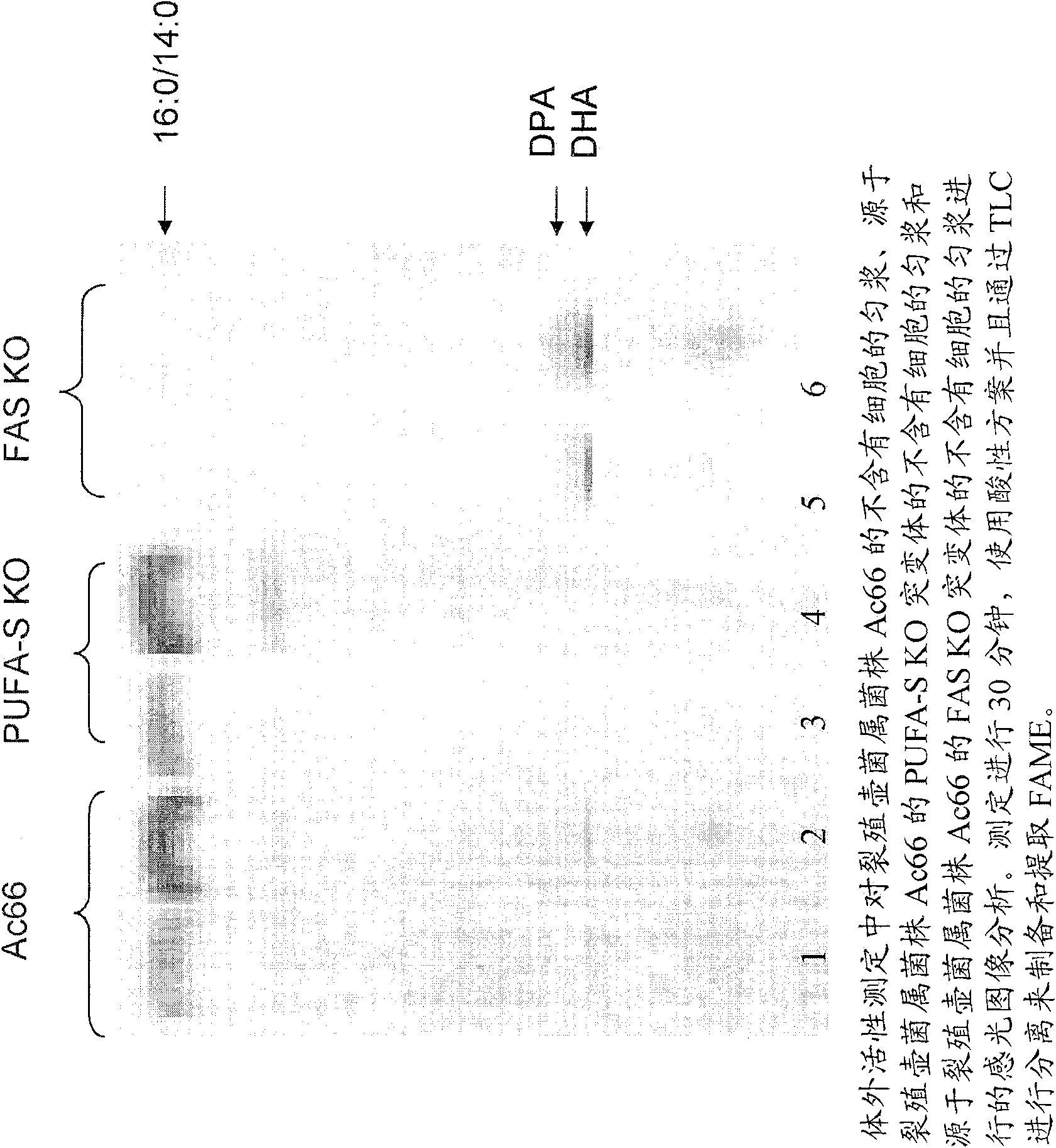
[0436] This example describes the generation of Schizochytrium FAS knockout strains for biochemical studies.
[0437] Schizochytrium contains a large gene encoding the FAS enzyme responsible for the production of short-chain saturated fatty acids (described in US Patent Application Publication No. 20050191679A1 ). Schizochytrium FAS knockout (FAS-KO) constructs were obtained using the methods described in US Patent No. 7,001,772. The ~10.0 kB EcoRV fragment in genomic DNA containing the majority of the FAS Orf (from ~728 bp downstream of the putative ATG start codon to ~680 bp downstream of the stop codon) was cloned into Stratagene Bluescript In the vector (pBSK), the vector is located at the EcoRV site of the multiple cloning region. The approximately 3.5 kB internal BglII fragment was removed from the cloned Schizochytrium DNA and replaced with the approximately 1.1 kB BamHI fragment from pTubZeo11-2 (which contains a Zeocin resistance cassette) (see the aforementioned US ...
Embodiment 2
[0439] The following examples describe general protocols for the preparation of cell-free extracts of the following strains: Schizochytrium Ac66, the PUFA synthase KO strain derived from Schizochytrium Ac66, and the PUFA synthase derived from Schizochytrium Ac66 FAS-KO strain.
[0440] An example of a protocol for preparing a cell-free homogenate (CFH) from a cell wall deficient strain of Schizochytrium is as follows. Cells were grown in A50-3 medium and then diluted into M2B medium. The medium used to grow the KO strains was supplemented with appropriate fatty acids. Cells were grown in M2B medium to an OD600nm > about 2.5 and < about 5. Cells in 50 mL of medium were collected by centrifugation in a 50 mL plastic tube (tabletop centrifuge, about 1200 rpm x 4 minutes). Decant the supernatant, then resuspend the cells in 5 mL of Buffer A (BufferA) (100 mM phosphate (pH 7.2), 10% (w / v) glycerol, 1 mM EDTA, and 2 mM DTT), and then Centrifuge as described above. Discard the s...
Embodiment 3
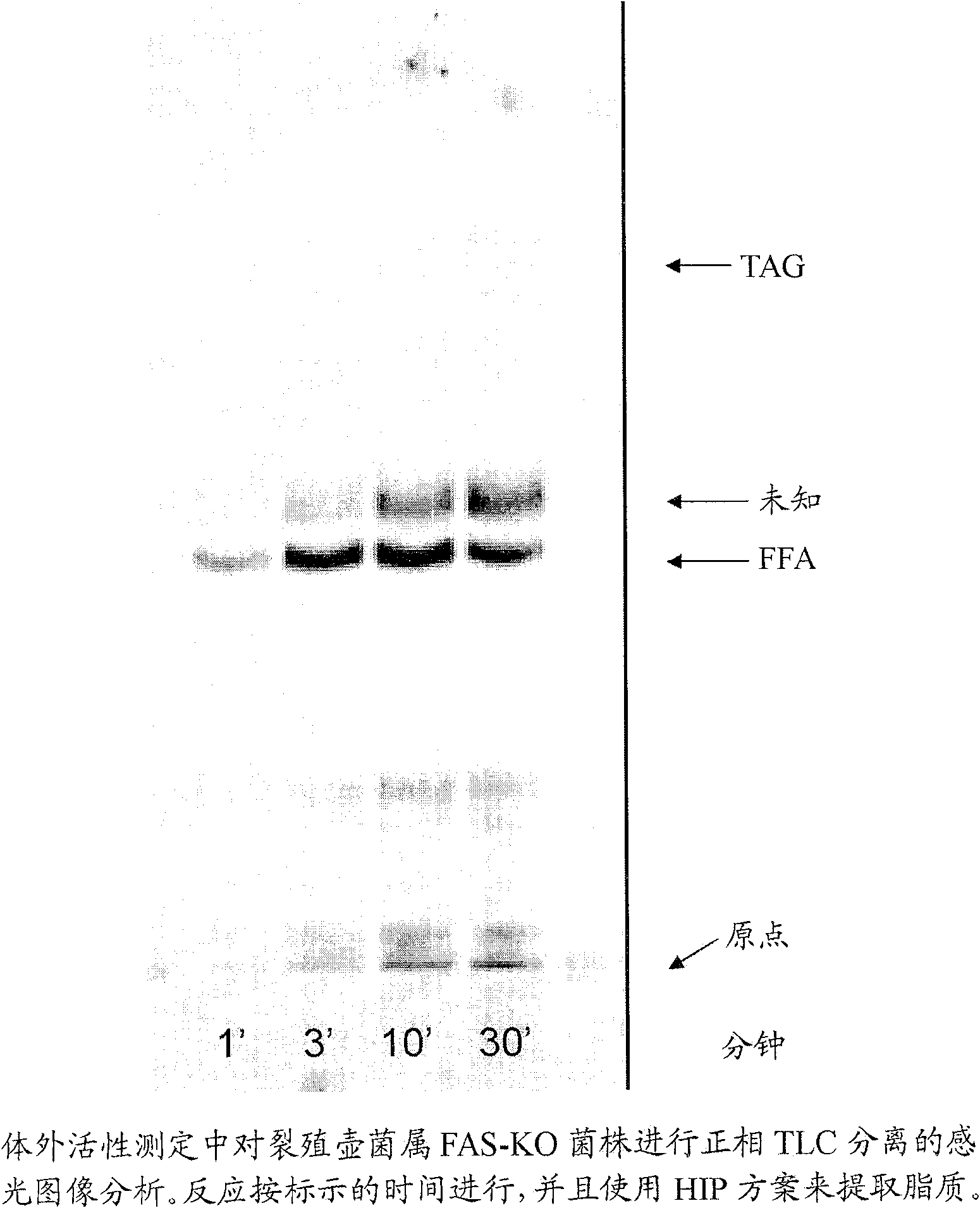
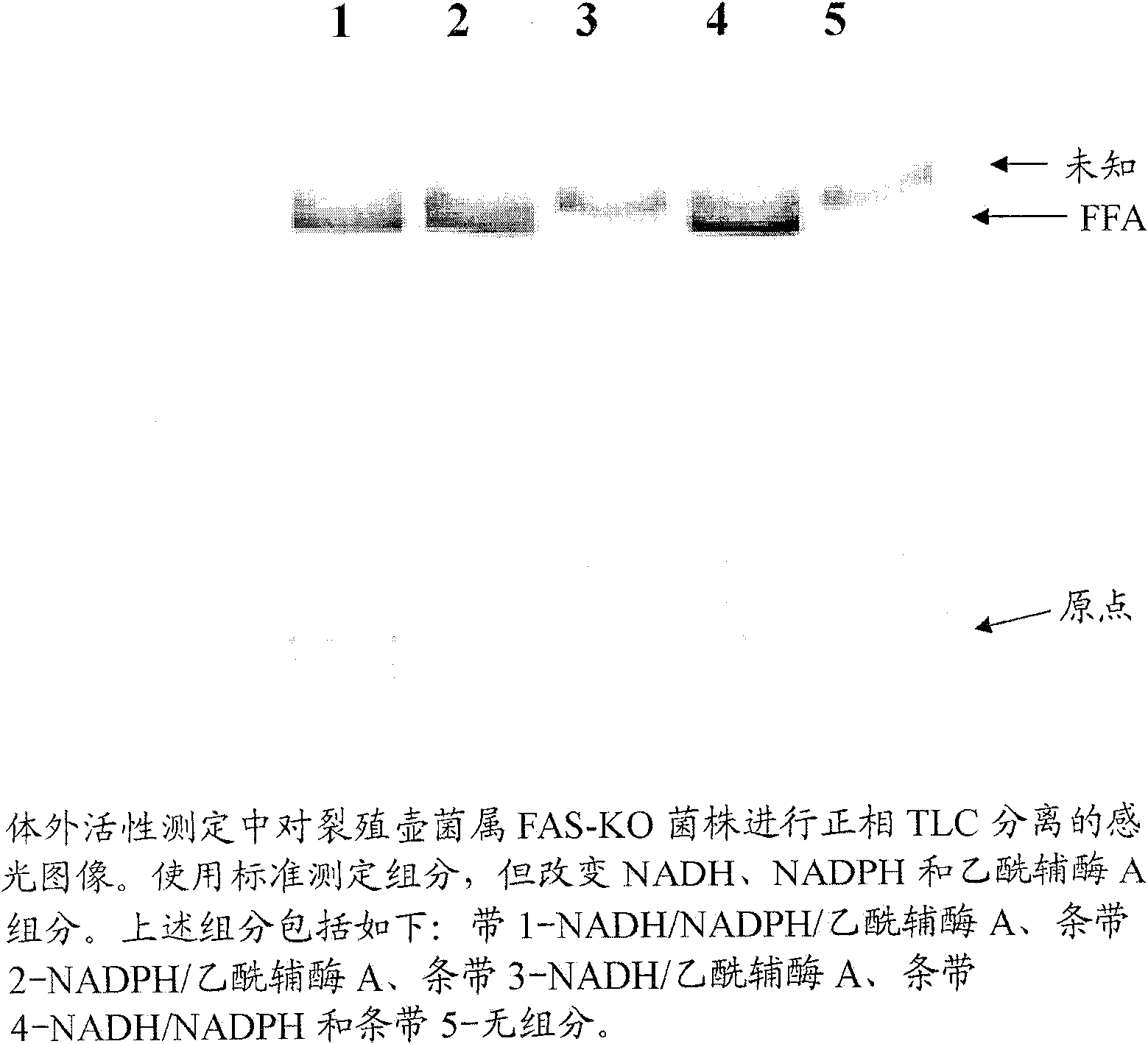
[0442] This example describes general conditions for in vitro FAS and PUFA synthetase activity assays.
[0443] Examples of protocols for in vitro activity assays for FAS and PUFA synthase activity are as follows. The enzyme preparation and buffer A (both components in a volume of 90 μL) and the following components (added as a cocktail (10 μL)) were mixed to a final volume of 100 μL to obtain the Final Concentration: Malonyl-CoA (50 μM - non-radioactive malonyl-CoA and malonyl-2- 14 A mixture of C-CoA with radiolabeled at a final concentration of 0.65 μCi / mL), NADH (1 mM), NADPH (1 mM) and acetyl-CoA (10 μM). These and additional components can be adjusted based on the needs of a particular experiment. The assay reactions were carried out in glass tubes in a water bath at room temperature (about 21°C). The time of incubation depends on the needs of the experiment. The reaction was stopped using one of two methods based on a work-up protocol. For the conversion of fatty a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com