Method for preparing lithium iron phosphate precursor comprehensively from laterite type nickel ores
A technology of lithium iron phosphate and laterite nickel ore, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., to achieve simple process flow, low cost, and good product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
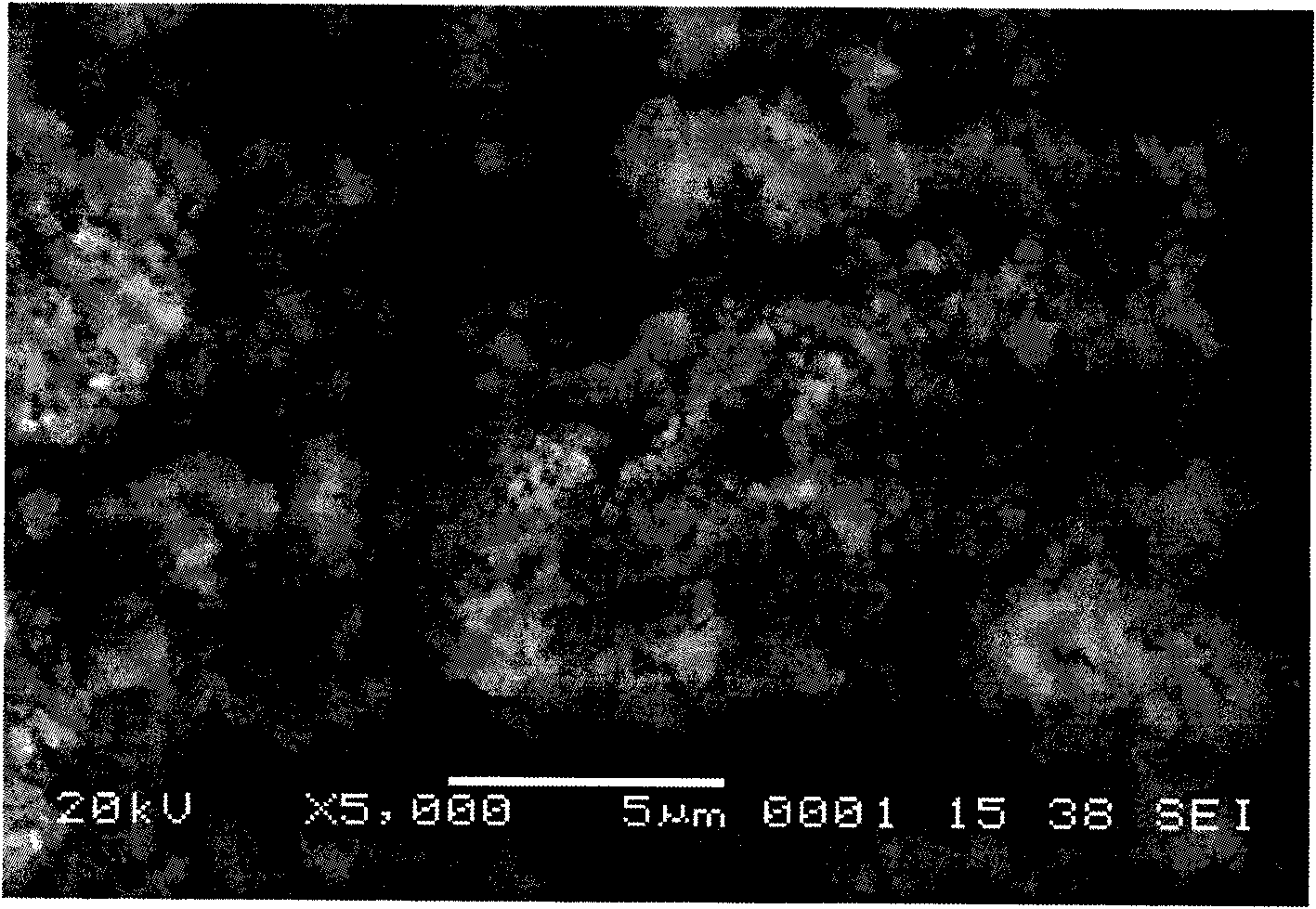
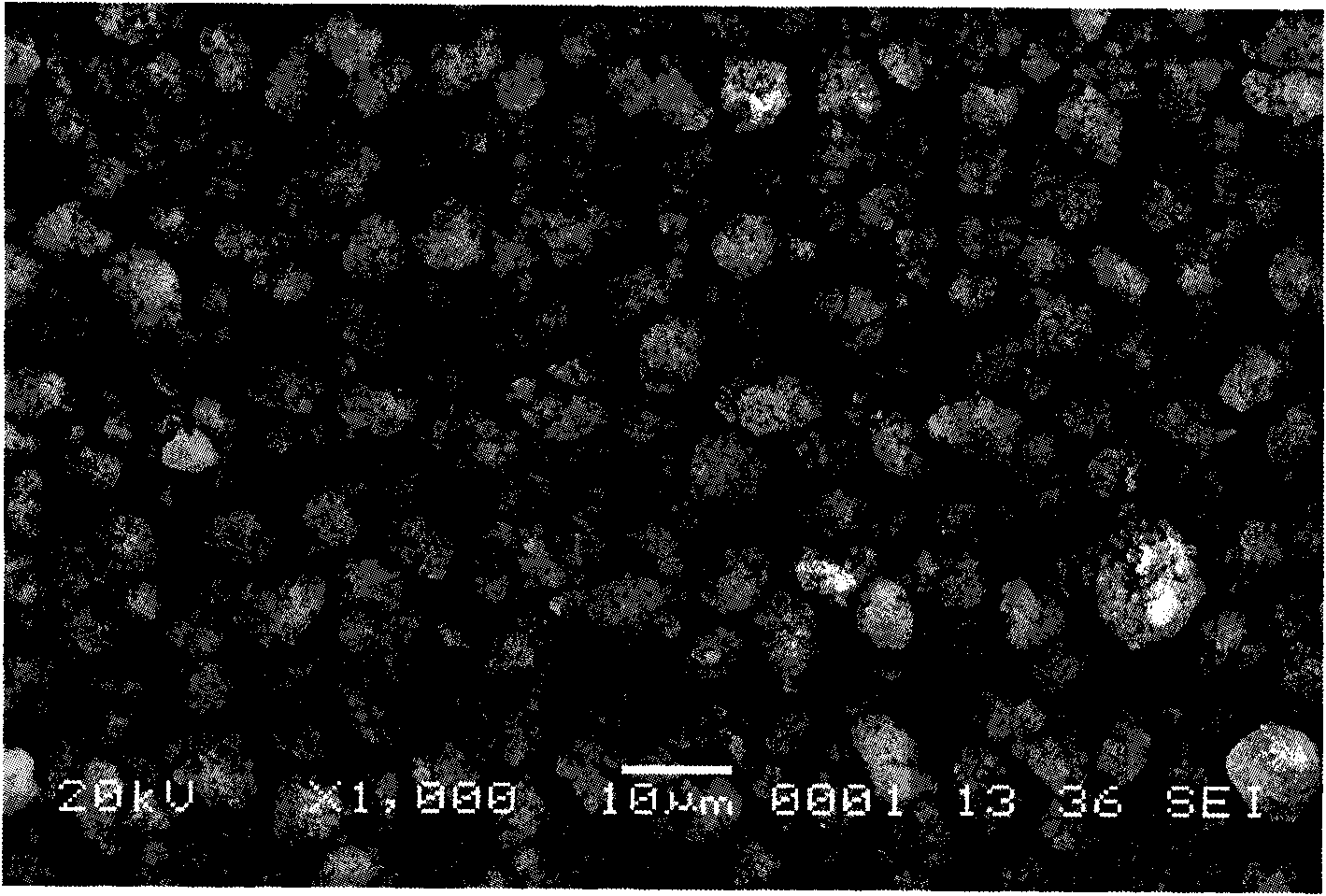
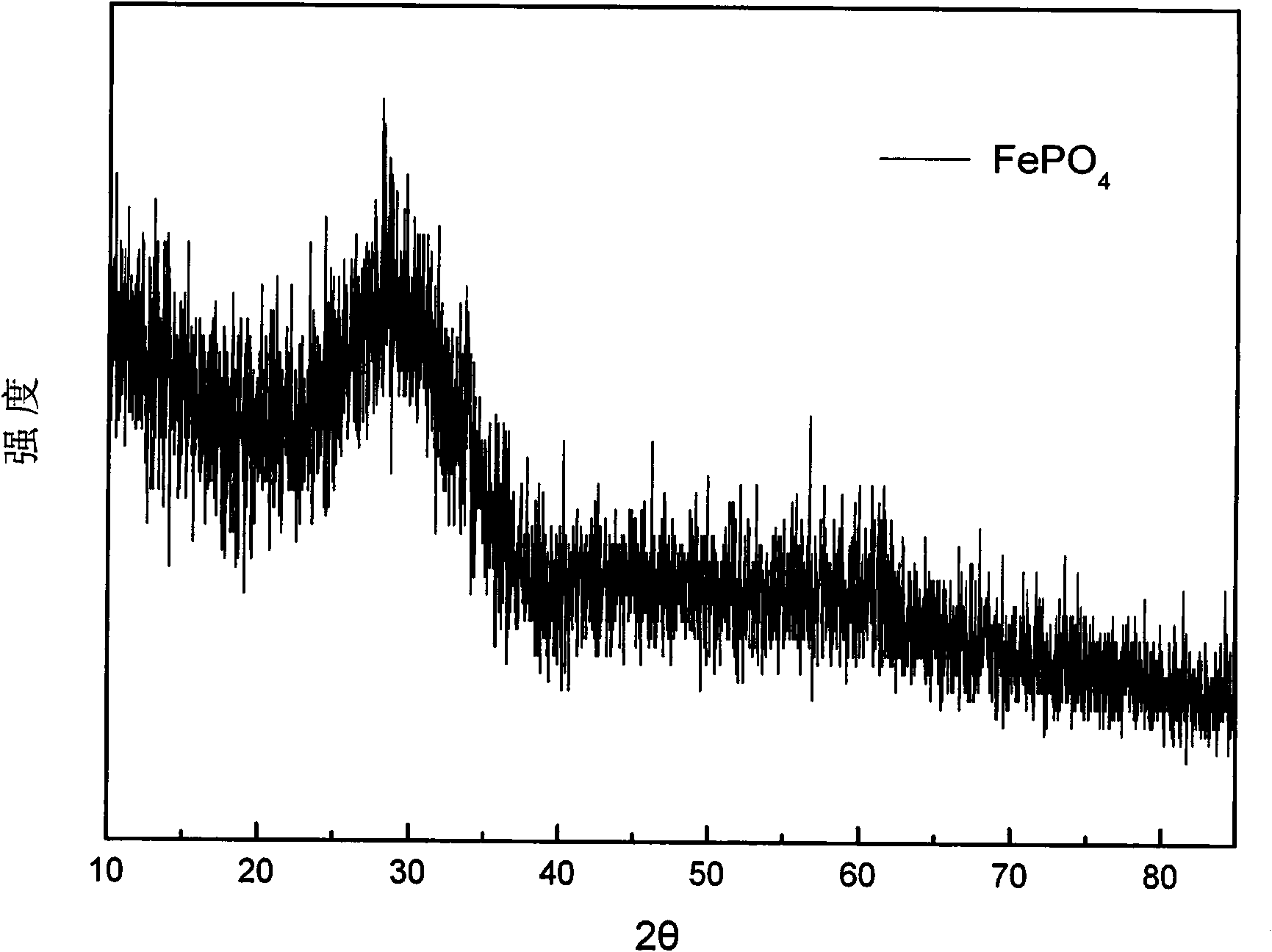
[0029] 500 grams of laterite nickel ore are leached with hydrochloric acid, so that the concentration of Fe in the leach solution is 0.5mol / L, adding sodium peroxide solution (1mol / L) in the solution, then adding phosphoric acid (1mol / L) equimolar with Fe, Use polyacrylic acid (0.01mol / L) to control the morphology, adjust pH=2.5±0.1 with sodium hydroxide solution (0.5mol / L), react in a stirred reactor at 40°C for 5min, wash and filter the resulting precipitate, and Dry at 100°C to obtain the precursor of lithium iron phosphate, the anode material of lithium ion battery, the mixture of iron phosphate and doped phosphate. The following table is the molar ratio of each element in the precursor of this embodiment.
[0030]
Embodiment 2
[0032] 500 grams of laterite nickel ore are leached with sulfuric acid, so that the concentration of Fe in the leach solution is 0.01mol / L, sodium hypochlorite solution (3mol / L) is added in the solution, and then triammonium phosphate solution (0.1mol / L) is added with Fe equimolar ), use citric acid (0.5mol / L) to control the morphology, adjust pH=2.5±0.1 with lithium hydroxide solution (2mol / L), react in a stirred reactor at 60°C for 24h, wash and filter the resulting precipitate, Dry at 50°C to obtain the precursor of lithium iron phosphate, the anode material of the lithium ion battery, the mixture of iron phosphate and doped phosphate.
Embodiment 3
[0034] 500 grams of laterite nickel ore are leached with hydrochloric acid and sulfuric acid mixed, so that the concentration of Fe in the leach solution is 2mol / L, potassium chlorate solution (0.01mol / L) is added in the solution, and then the ammonium dihydrogen phosphate solution ( 9mol / L), control the morphology with tetraethylethylene glycol (0.01mol / L), adjust the pH=3.0±0.1 with ammonia water (0.01mol / L), react in a stirred reactor at 20°C for 10h, and prepare the obtained The precipitate is washed, filtered, and dried at 200°C to obtain a mixture of iron phosphate and doped phosphate, which is the precursor of lithium iron phosphate, the positive electrode material of lithium ion batteries.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com