2beta-chloropodophyllotoxin aromatic acid ester compounds, preparation and application in preparing botanical pesticide
A technology of chloropodophyllotoxins and aromatic acid esters, applied in the directions of plant growth regulators, applications, biocides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1. Products: 2β-chloropodophyllotoxin and compounds 1-8 The physical and chemical properties of each compound are introduced in detail in the following content.
[0027] 2. Preparation method:
[0028] The following is the synthetic route of 4-O-tetrahydropyranopodophyllotoxin:
[0029] Weigh 1 mmol of podophyllotoxin and dissolve it in 10 mL of dihydropyran, add dropwise 1 drop of phosphorus oxychloride, dissolve in a water bath at 60-63°C, stir the reaction at room temperature, and track and detect it by TLC. When the reaction is almost complete, the unreacted dihydropyran is evaporated to dryness under reduced pressure, and the pure product is obtained by silica gel column chromatography.
[0030]
[0031] Podophyllotoxin 4-O-tetrahydropyranopodophyllotoxin
[0032] The physical and chemical properties of 4-O-tetrahydropyranopodophyllotoxin are as follows:
[0033] 1), white solid, melting point 93-94°C.
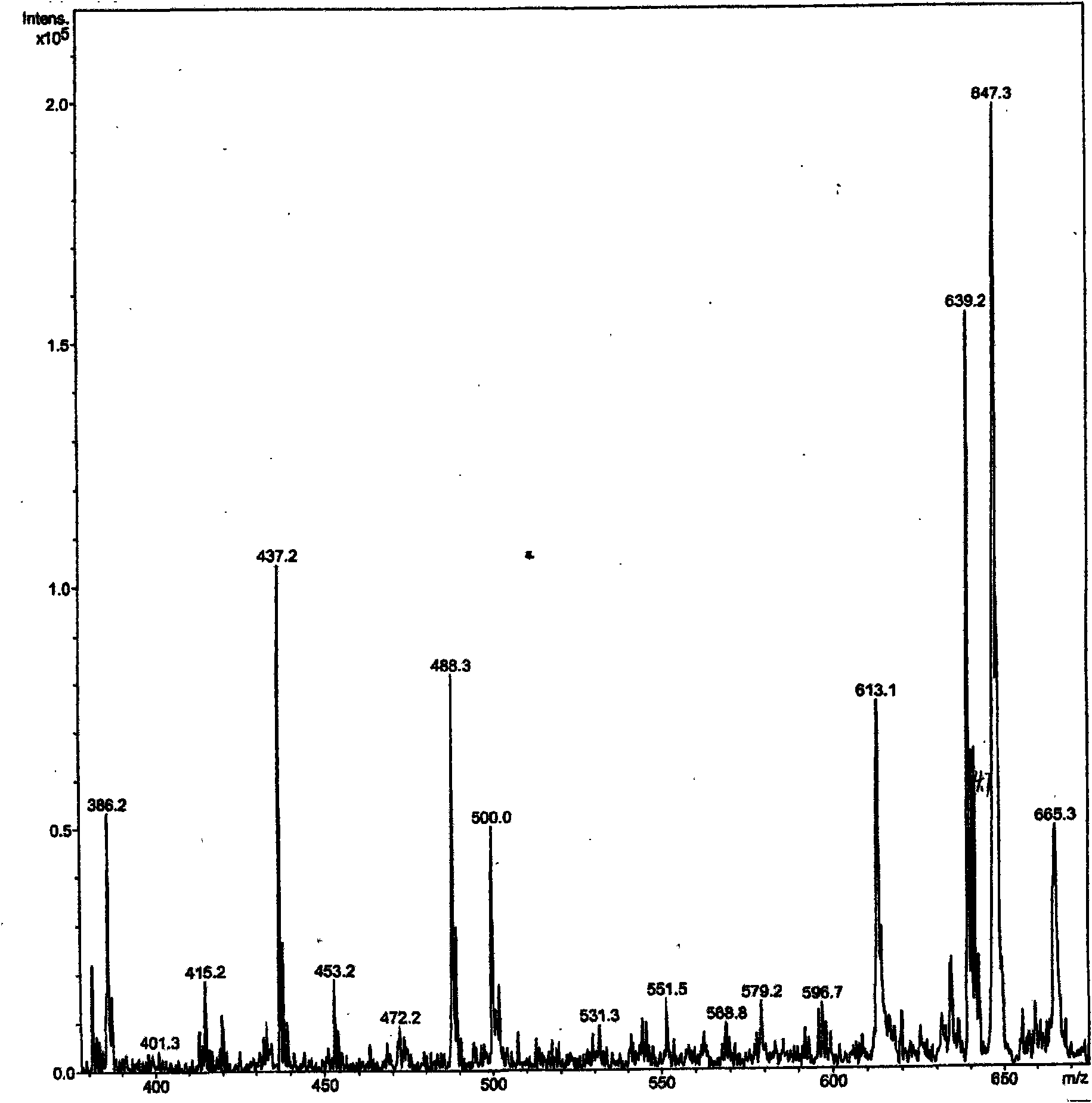
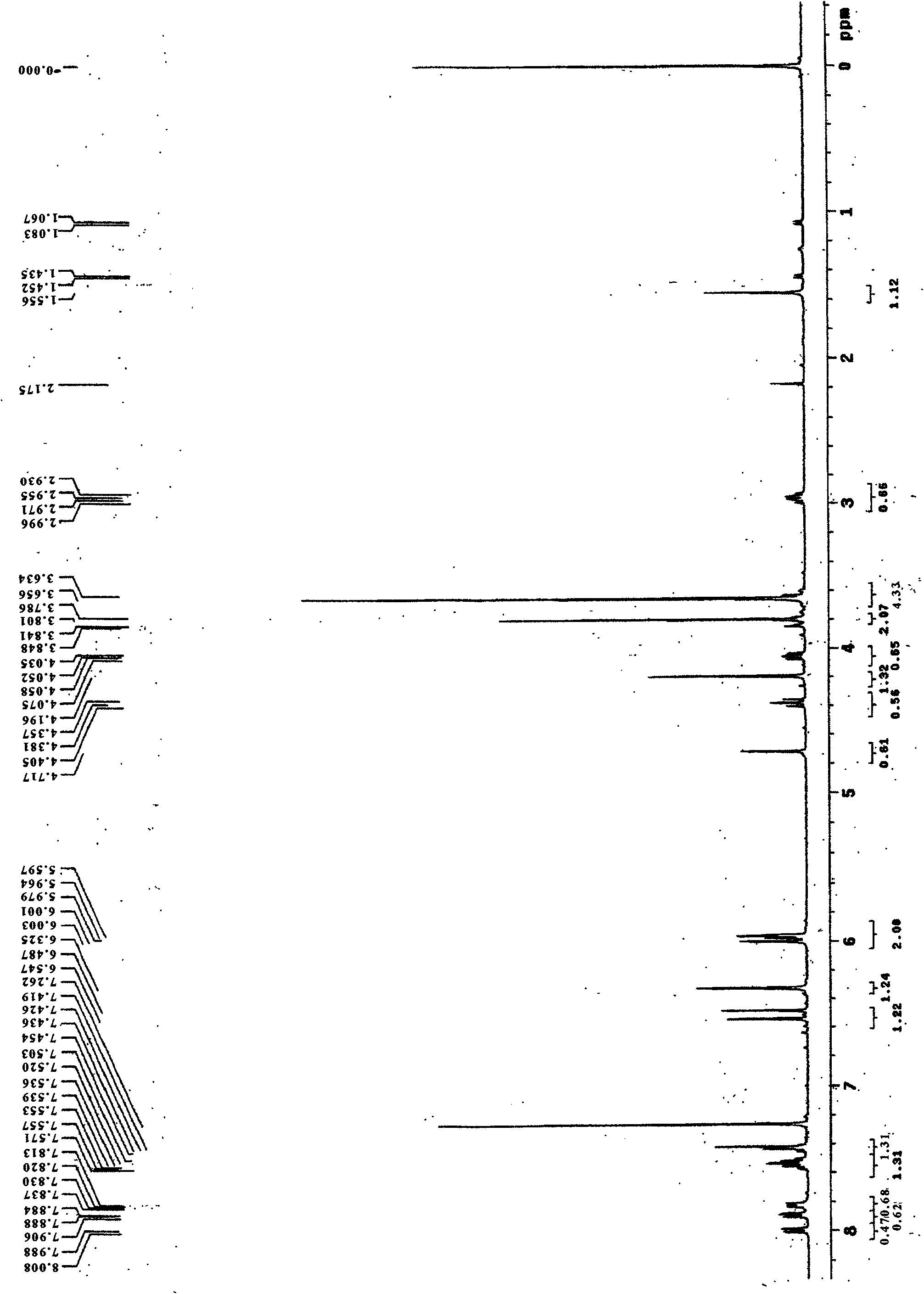
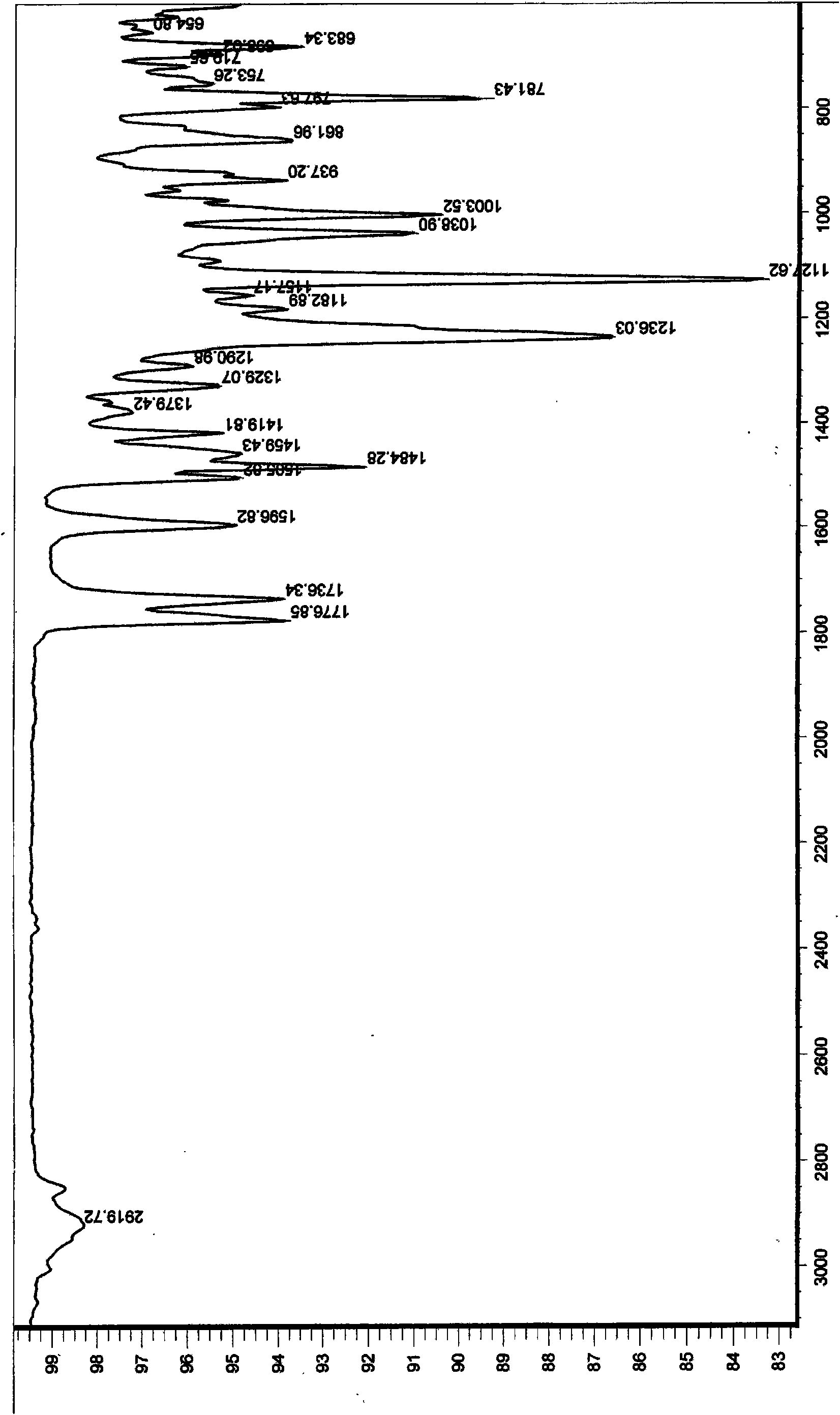
[0034] 2), 4-O-tetrahydropyranopodophyllotoxin infrare...
Embodiment 2
[0147] Bioassay experiment of the present invention:
[0148] 1. Insects to be tested: armyworm larvae in the early 3rd instar, provided by the insect breeding room of the Pollution-free Pesticide Research Center of Northwest A&F University.
[0149] 2. Samples and reagents:
[0150] The samples are: toosendanin, podophyllotoxin, 4-O-tetrahydropyranopodophyllotoxin, 4-O-tetrahydropyrano-2β-chloropodophyllotoxin, 2β-chloropodophyllotoxin and examples 1 Compounds 1-8 prepared. The solvent was acetone, analytically pure, from Chengdu Kelong Chemical Reagent Factory.
[0151] 3. Bioassay method:
[0152] Using the method of adding small leaf butterfly: spread a layer of filter paper on the bottom of a petri dish with a diameter of 9 cm, and add water to moisturize it. Pick 10 3rd instar prophase armyworm larvae with the same size and robustness from each dish. Weigh 5 mg toosendanin, podophyllotoxin, 4-O-tetrahydropyranopodophyllotoxin, 4-O-tetrahydropyrano-2β-chloropodophyll...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com