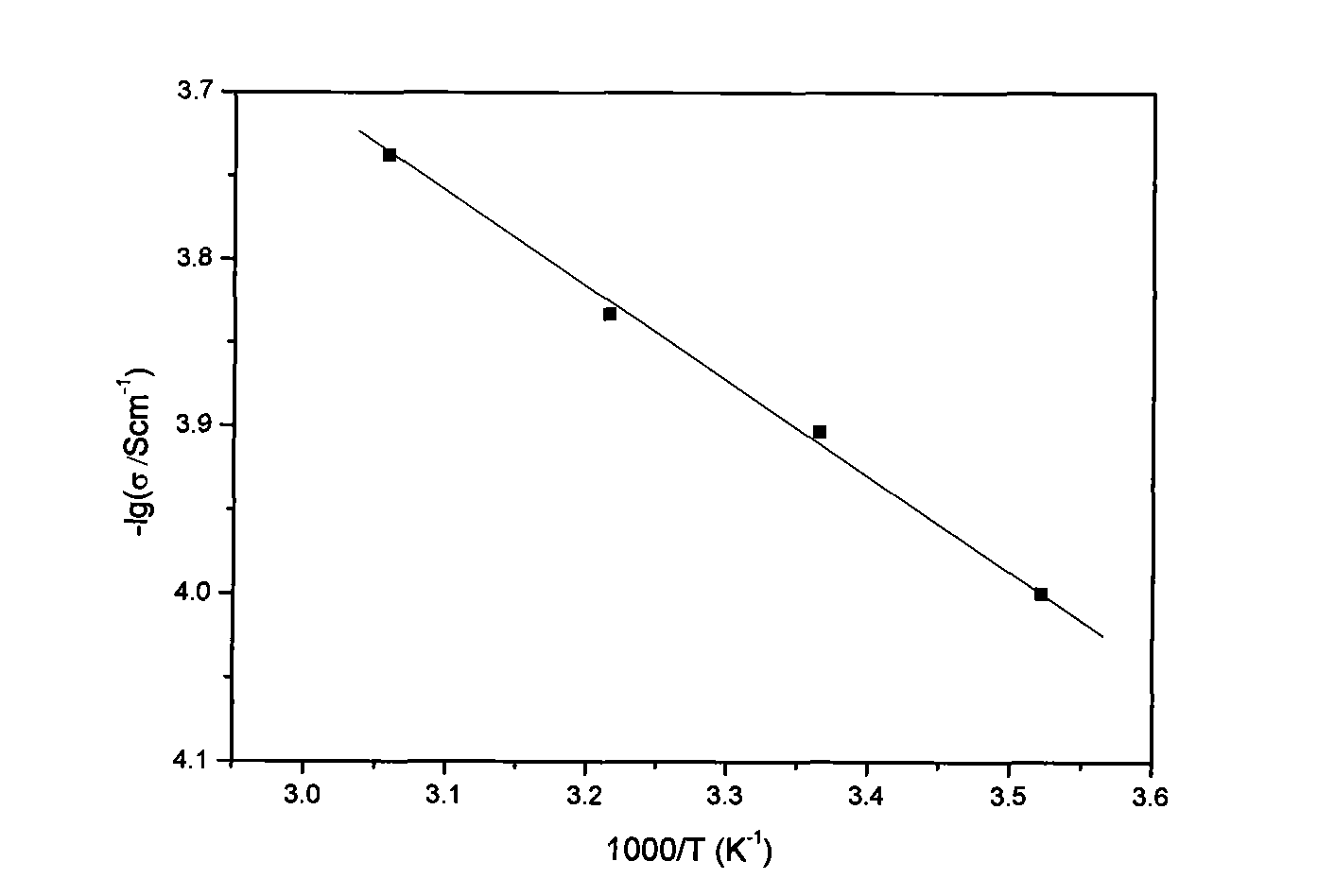
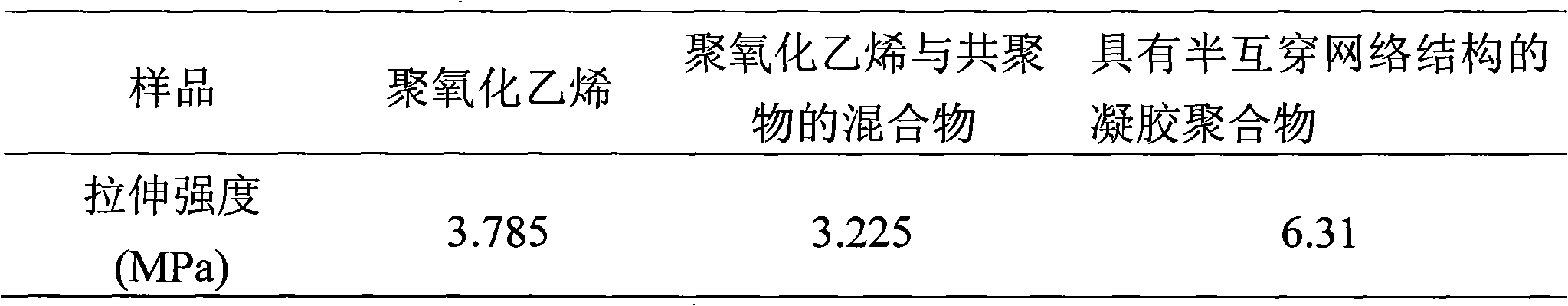
Gel polymer electrolyte with semi-interpenetrating network structure and preparation method thereof
A gel polymer and network structure technology, applied in circuits, electrical components, secondary batteries, etc., can solve rare problems and achieve the effects of inhibiting crystallization, improving mechanical properties, and high ion conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 1) Add 0.53g of acrylonitrile, 2.12g of glycidyl methacrylate, 0.015g of benzoyl peroxide and 40ml of N,N-dimethylformamide into a three-necked flask. After it was completely dissolved, it was reacted at 60° C. for 10 h under the protection of nitrogen. The resulting product was precipitated with methanol, dissolved in acetone, and precipitated with methanol, repeated three times. The product was dried in a vacuum oven at 40° C. to a constant weight to obtain a copolymer.
[0022] 2) Dissolve 0.05g of copolymer and 0.2g of polyethylene oxide into 50ml of acetonitrile solution under stirring at room temperature; after the above solution is completely dissolved, add 0.004g of diethylenetriamine and crosslink at 60°C for 4 hours; dissolve the above solution Cast on a polytetrafluoroethylene plate, volatilize at room temperature to form a film, then place it in a vacuum oven at 70°C for 12 hours to make the system completely cross-linked, and immerse the obtained film in 1m...
Embodiment 2
[0024] 1) Add 0.53g of acrylonitrile, 2.84g of glycidyl methacrylate, 0.03g of benzoyl peroxide and 50ml of N,N-dimethylformamide into a three-necked flask successively. After it was completely dissolved, it was reacted at 70° C. for 10 h under the protection of nitrogen. The resulting product was precipitated with methanol, dissolved in acetone, and precipitated with methanol, repeated three times. The product was dried in a vacuum oven at 40° C. to a constant weight to obtain a copolymer.
[0025] 2) Dissolve 0.2g of copolymer and 0.4g of polyethylene oxide into 50ml of acetonitrile solution under stirring at room temperature; after the above solution is completely dissolved, add 0.02g of diethylenetriamine to crosslink at 80°C for 2 hours; cast the above solution Put it on a polytetrafluoroethylene plate, volatilize at room temperature to form a film, put it in a vacuum oven at 70°C for 12 hours, make the system cross-linked completely, and immerse the obtained film in 1mol...
Embodiment 3
[0027] 1) Add 0.53g of acrylonitrile, 3.18g of glycidyl methacrylate, 0.037g of benzoyl peroxide and 50ml of N,N-dimethylformamide (DMF) into a three-necked flask successively. After it was completely dissolved, it was reacted at 80° C. for 6 h under the protection of nitrogen. The resulting product was precipitated with methanol, dissolved in acetone, and precipitated with methanol, repeated three times. The product was dried in a vacuum oven at 40° C. to a constant weight to obtain a copolymer.
[0028] 2) Dissolve 0.2g of copolymer and 0.3g of polyethylene oxide into 50ml of acetonitrile solution under stirring at room temperature; after the above solution is completely dissolved, add 0.03g of diethylenetriamine to crosslink at 70°C for 3 hours; cast the above solution Put it on a polytetrafluoroethylene plate, volatilize at room temperature to form a film, put it in a vacuum oven at 70°C for 12 hours, make the system cross-linked completely, and immerse the obtained film i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com