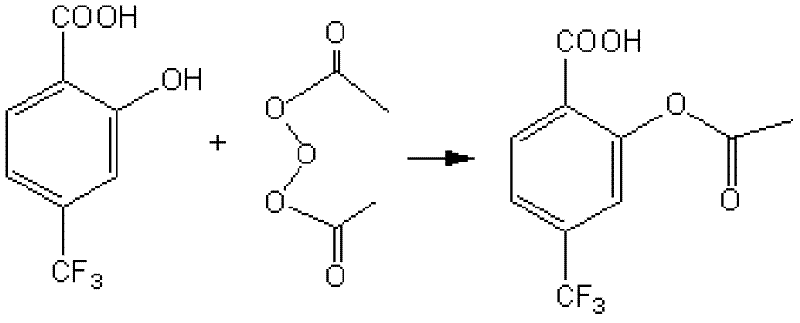
Preparation method of officinal 4-trifluoromethyl acetylsalicylic acid
A technology of trifluoromethyl acetylsalicylic acid and trifluoromethyl salicylic acid, applied in the field of medicine, can solve the problem that the product purity is difficult to meet the purity requirements for medicinal use, and achieves the advantages of large-scale production operation, quality Stable, easy-to-use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Put 20g of 4-trifluoromethylsalicylic acid and 50g of acetic anhydride into the reaction axe, start stirring, and slowly add 3g of concentrated sulfuric acid dropwise. After the dropwise addition, slowly raise the temperature to 30°C, and keep it warm for 3 hours, add the reaction liquid dropwise into 100g of water, stir, and a white solid precipitates, continue to stir for 1h after the dropwise addition, suction filter, and wash with a large amount of deionized water Filter cake until the pH reaches about 7 to obtain white crystals, and vacuum-dry at 45° C. for 10 hours to obtain 15.0 g of crude 4-trifluoromethylacetylsalicylic acid.
[0031] Add 15.0g of the crude product into the reaction flask, add 45g of petroleum ether (60-90°C), add 15g of ethyl acetate, heat to reflux, the crude product is basically completely dissolved, filter out a small amount of insoluble matter, naturally cool to room temperature for crystallization, cool to After room temperature, crystall...
Embodiment 2
[0038] Put 20g of 4-trifluoromethylsalicylic acid and 50g of acetic anhydride into the reaction axe, start stirring, and slowly add 3g of concentrated sulfuric acid dropwise. After the dropwise addition, slowly raise the temperature to 30°C, and keep it warm for 2 hours, add the reaction liquid dropwise into 100g of water, stir, and a white solid precipitates, continue to stir for 1h after the dropwise addition, filter with suction, and wash with a large amount of deionized water Filter cake until the pH reaches about 7 to obtain white crystals, and vacuum-dry at 45° C. for 10 hours to obtain 15.2 g of crude 4-trifluoromethylacetylsalicylic acid.
[0039] Add 15.2g of the crude product to the reaction flask, add 60g of petroleum ether (60-90°C), add 15g of ethyl acetate, heat to reflux, the crude product is basically completely dissolved, filter out a small amount of insoluble matter, naturally cool to room temperature for crystallization, cool to After room temperature, cryst...
Embodiment 3
[0046] Put 20g of 4-trifluoromethylsalicylic acid and 50g of acetic anhydride into the reaction axe, start stirring, and slowly add 3g of concentrated sulfuric acid dropwise. After the dropwise addition, slowly raise the temperature to 30°C, and keep it warm for 2 hours, add the reaction liquid dropwise into 100g of water, stir, and a white solid precipitates, continue to stir for 1h after the dropwise addition, filter with suction, and wash with a large amount of deionized water Filter cake until the pH reaches about 7 to obtain white crystals, and vacuum-dry at 45° C. for 10 hours to obtain 14.9 g of crude 4-trifluoromethylacetylsalicylic acid.
[0047] Add 14.9g of the crude product to the reaction flask, add 60g of petroleum ether (60-90°C), add 30g of ethyl acetate, heat to reflux, the crude product is basically completely dissolved, filter out a small amount of insoluble matter, naturally cool to room temperature for crystallization, and cool to After room temperature, c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com