Method for preparing magnetic oxide iron and magnetic oxide iron desulfurizer prepared thereby
A technology of magnetic iron oxide and desulfurizer, applied in the direction of iron oxide/hydroxide, etc., can solve the problems of undisclosed and undisclosed preparation methods of magnetic iron oxide, and achieve the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
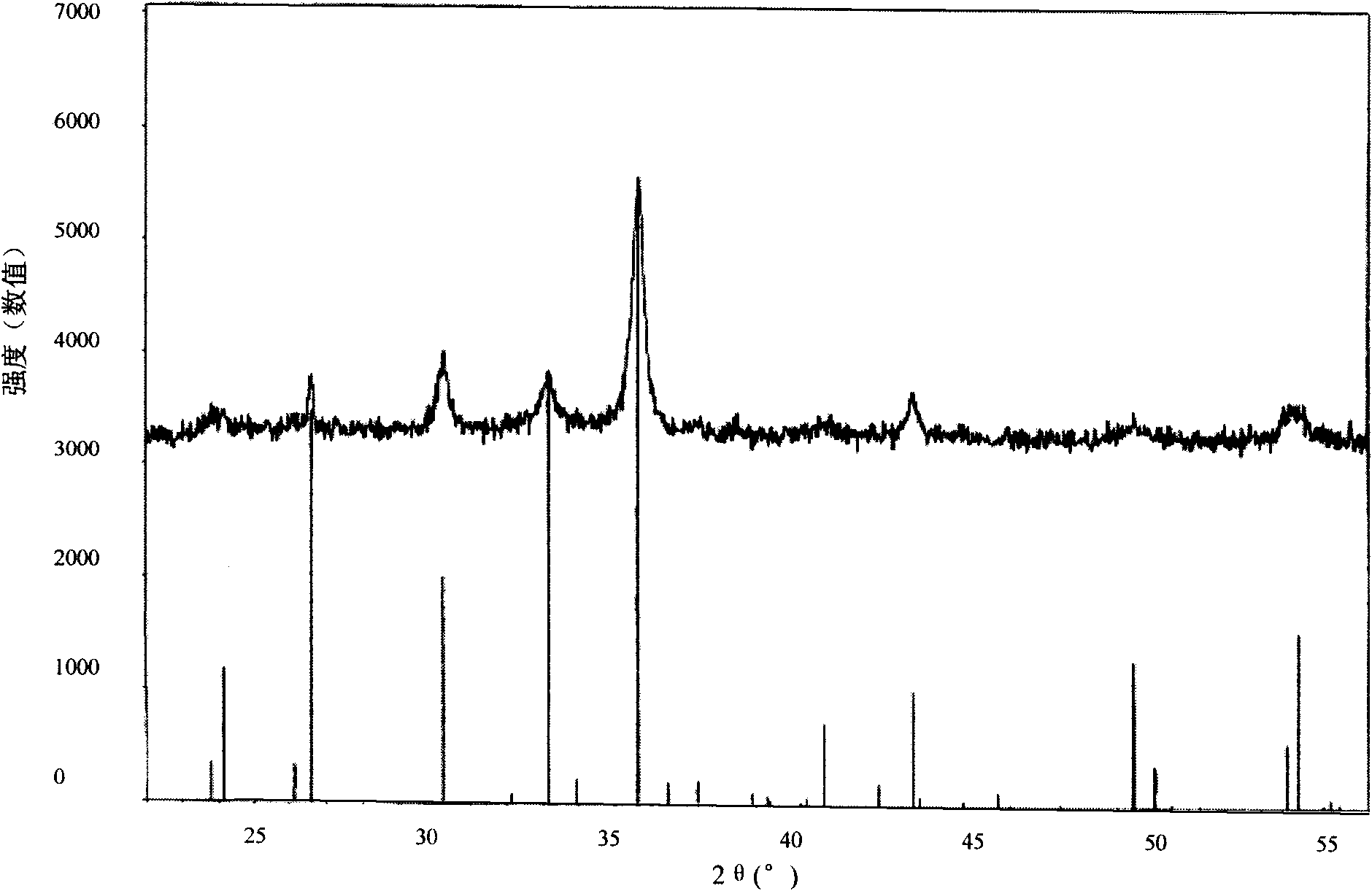
Image
Examples
Embodiment 1
[0019] Mix 32g of ferrous sulfate powder and 12g of sodium hydroxide evenly, wherein the molar ratio of iron to hydroxide is 1:2.8; put the above mixed material into a kneader, and knead for 3 hours to complete the solidification between the above reactants. Reaction, then the above reaction product is placed in the air to dry, in order to facilitate the full completion of the above reaction.
[0020] Stir the dried material with water first, and wash repeatedly until the obtained filtrate has no sulfate radicals (usually tested with barium chloride), and then use a centrifuge to filter the above washing liquid.
[0021] Dry the above-mentioned washed and filtered solid at a drying temperature of 80°C and a drying time of 3 hours to obtain amorphous iron oxyhydroxide, and roast the above-mentioned amorphous iron oxyhydroxide at 200°C for 2 hours to obtain Obtain the magnetic iron oxide Fe described in the present invention 21.333 o 32 Desulfurizer A.
[0022] In this desulf...
Embodiment 2
[0024] Mix 64g of ferrous sulfate powder and 21.2g of sodium hydroxide evenly, wherein the molar ratio of iron to hydroxide is 1:2.4; put the above mixed material into a kneader and knead for 0.5h to complete the reaction between the above reactants. The solid-phase reaction was carried out, and then the above reaction product was air-dried.
[0025] Add water and stir the dried material first, and wash repeatedly until the obtained filtrate has no sulfate radicals (usually tested with barium chloride), and use a centrifuge to filter the above washing liquid.
[0026] Naturally dry the above-mentioned washed and filtered solid, the drying temperature is -5°C, and the drying time is 10h, then the amorphous iron oxyhydroxide can be obtained, and the above-mentioned amorphous iron oxyhydroxide is roasted at 190°C for 1.0h. Obtain the magnetic iron oxide Fe described in the present invention 21.333 o 32 Desulfurizer B.
[0027] In this desulfurizer B, containing 97.0wt% magneti...
Embodiment 3
[0029] Mix 34.2g of ferrous nitrate powder and 23.4g of potassium hydroxide evenly, wherein the molar ratio of iron to hydroxide is 1:2.0; put the above mixed material into a kneader and knead for 1.0h to complete the reaction of the above reactants The solid phase reaction between them was carried out, and then the above reaction product was air-dried.
[0030] Add water and stir the dried material first, and wash repeatedly until the obtained filtrate has no sulfate radicals (usually tested with barium chloride), and use a centrifuge to filter the above washing liquid.
[0031] Naturally dry the above-mentioned washed and filtered solid at a drying temperature of 45°C and a drying time of 3 hours to obtain amorphous iron oxyhydroxide, and roast the above-mentioned amorphous iron oxyhydroxide at 180°C for 2.0 hours to obtain Obtain the magnetic iron oxide Fe described in the present invention 21.333 o 32 Desulfurizer C.
[0032] In this desulfurizer C, containing 93.1wt% m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com