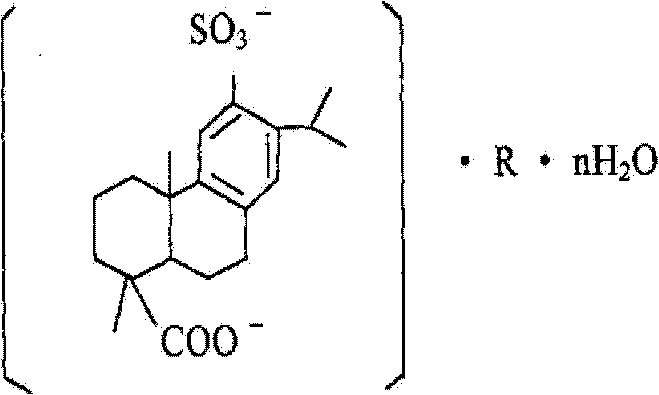
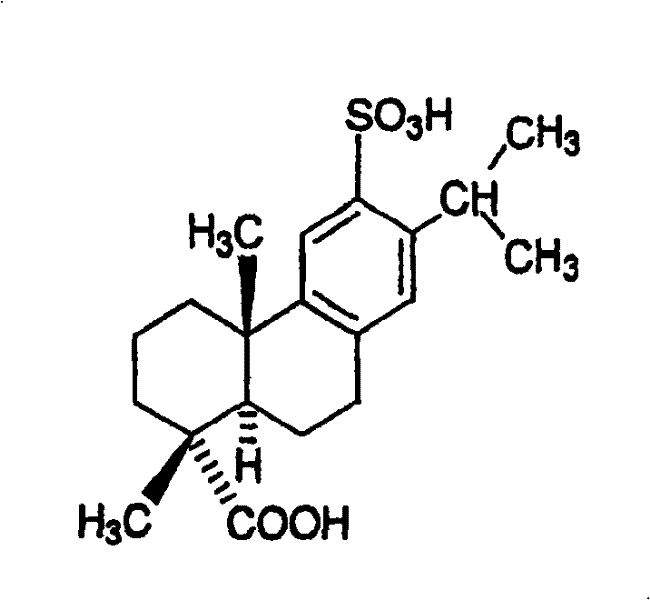
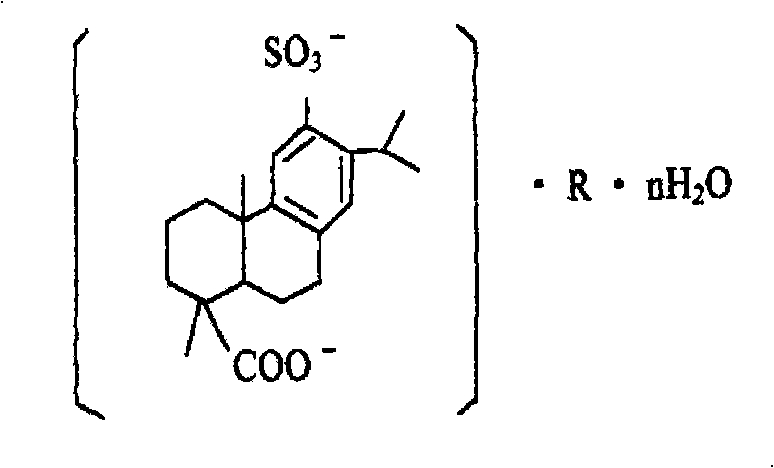
preparation method of disodium sulfodehydroabietate (DSDA) and composition
A technology of disodium abietic acid and dehydroabietic acid, which is applied in the direction of drug combination, medical preparations containing active ingredients, digestive system, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0203] Preparation of disodium sulfonated dehydroabietic acid:
[0204] (1) Preparation of abietic acid
[0205] Take 5 kg (about 15.5 mol) of golden yellow 310 resin, that is, rosin calcium grease, crush it, place it in a three-necked flask equipped with a stirrer and a reflux condenser, add 3 L of industrial ethyl acetate (about 30.6 mol) and 600 ml of hydrochloric acid, Adjust the pH value to 3-4, heat and reflux under stirring, react for 1 hour, stop stirring and let stand for a while, so that the insoluble matter settles completely, pour out the supernatant while it is hot, and place the supernatant for crystallization (about 2 days). Filtrate, wash the filter cake with an appropriate amount of ethyl acetate, combine the lotion and filtrate, concentrate and recover about 1L of ethyl acetate, continue to place it for crystallization, crystallize and filter out, then wash with industrial ethyl acetate, dry the crystallization, and obtain crude abietic acid A total of 2225g...
Embodiment 2
[0215] Preparation of disodium sulfonated dehydroabietic acid granules, capsules and solutions:
[0216] (1) The weight proportion of combination and the binder selected in each prescription are listed in Table 8
[0217] The weight ratio of combination and the selected adhesive unit in each prescription of table 8: gram
[0218] Raw materials
Prescription 1
Prescription 2
Prescription 3
Prescription 4
Prescription 5
Prescription 6
Prescription 7
Disodium sulfonated dehydroabietic acid (calculated as anhydrous matter)
500
500
500
500
500
500
500
300
400
500
400
300
400
500
70
40
70
70
40
70
90
10% pvp
water
30% ethanol
70% ethanol
95% ethanol
10% pvp ethanol solution
5% pvp ethanol solution
[0219] (2) Pulverize disodium sulfonated...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com