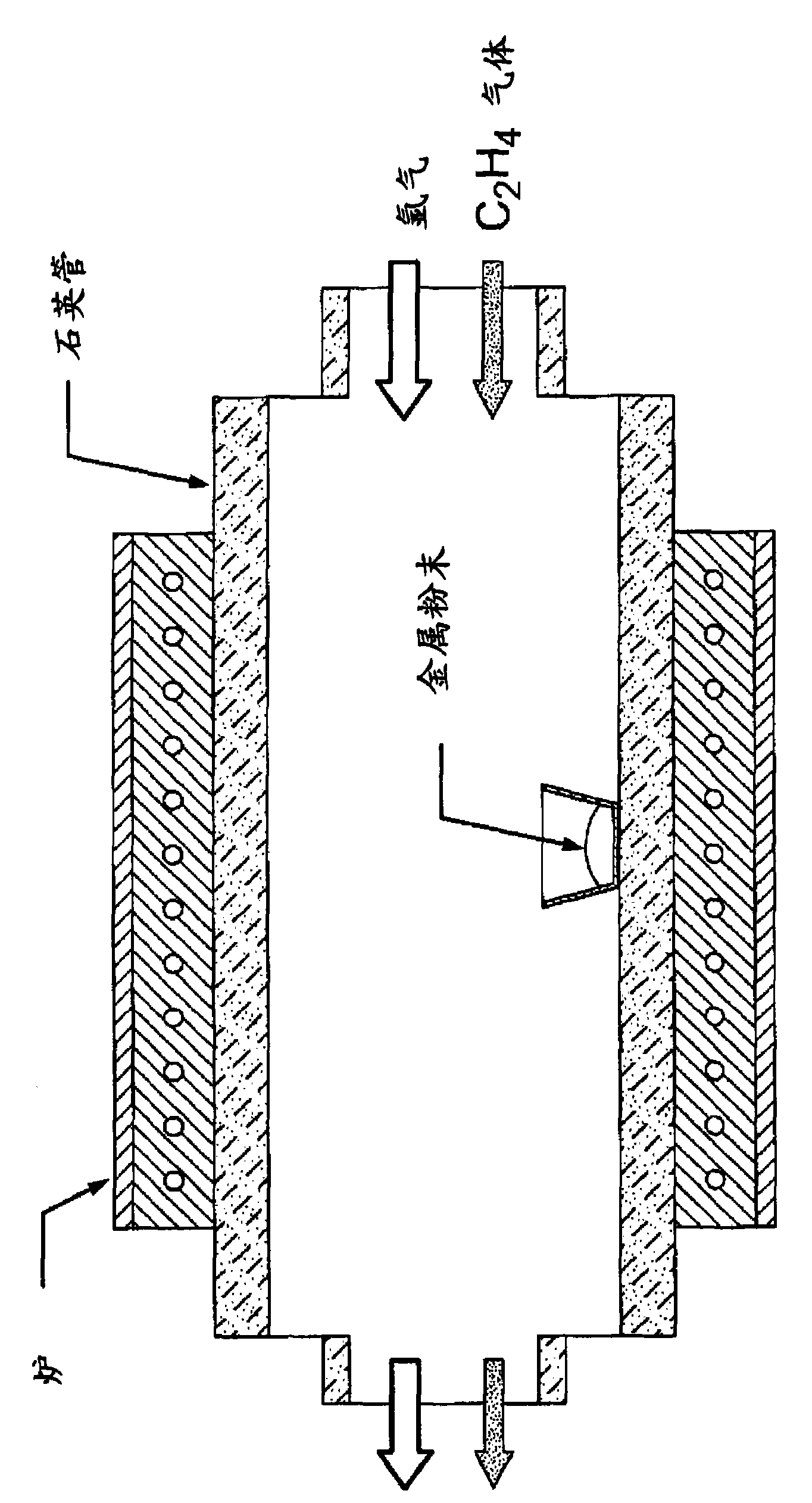
Nanowire supported catalysts for fuel cell electrodes
A supported catalyst and fuel cell technology, which is applied to fuel cell parts, fuel cells, battery electrodes, etc., can solve the problems that catalyst particles cannot effectively provide three-phase interface reaction sites and reduce the utilization rate of platinum materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1: SnO as an electrode 2 Nanowire-carbon paper composites:
[0056] This example demonstrates that nanowires can be grown directly on carbon paper and platinum particles, for example, can then be selectively deposited on the nanowires to improve platinum utilization by ensuring an electron path from the platinum particles to the supporting electrode in a PEM fuel cell. Rate. As described above in SnO 2 Platinum deposition on nanowires. But of practical importance is the demonstration that the nanowire-carbon paper composite is in fact an electrode, ie the nanowires are in electrical contact with the carbon fibers of the carbon paper.
[0057] by a standard three-electrode electrochemical cell at 0.5M K 2 SO 4 5mM K in 3 Fe(CN) 6 Cyclic Voltammetry Characterization of SnO in Aqueous Solution 2 Electrical contact between nanowires and carbon fibers of carbon paper. Working electrode is carbon paper or SnO 2 nanowire electrodes. An Ag / AgCl (3M KCl) elect...
Embodiment 2
[0058] Example 2: Characterization of deposited Pt nanoparticles by cyclic voltammetry:
[0059] 1. SnO loaded with Pt nanoparticles prepared by electrochemical method 2 nanowire catalyst
[0060] show electrodeposition on SnO 2 Pt nanoparticles on nanowires in Ar-saturated 0.5M H at room temperature 2 SO 4 Cyclic voltammogram data for the electrocatalytic properties in aqueous solution are presented in Figure 6A middle. The cyclic voltammetric performance is reproducible and representative of hydrogen absorption and desorption. A reduction peak centered at 0.5 V can be observed during the negative potential sweep ( Figure 6A ). This reduction peak can be attributed to the reduction of platinum oxide. This feature of the curve is consistent with the cyclic voltammogram of the Pt electrode. Therefore, it can also be concluded that platinum nanoparticles have extremely clean active surfaces. This result suggests that the thus deposited Pt nanoparticles exhibit higher...
Embodiment 3
[0068] Example 3: Electrocatalytic Activity for Oxygen Reduction Reaction in Acidic Solution
[0069] 1. Nanowire catalysts loaded with Pt nanoparticles prepared by electrochemical method:
[0070] Oxygen reduction reactions are especially important for achieving high-efficiency fuel cells, batteries, and many other electrode applications. Pt and Pt-based alloy particles on various carbon supports are the most widely used and efficient catalysts for fuel cell electrodes. For SnO using supported Pt nanoparticles 2 Oxygen reduction experiment of nanowire catalyst, 0.5M H 2 SO 4 The solution was purged with ultrapure oxygen for 30 minutes. The solution becomes fully oxygen saturated. at 50mVs -1 The scan rate scans the electrode over a potential range of 0.8 V to 0.2 V. Figure 7 is shown for comparison of SnO 2 Voltammetric data for the oxygen reduction reaction current of the nanowires (curve A). Curve B shows the SnO 2 Voltammetry data for nanowire-supported Pt nanop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com