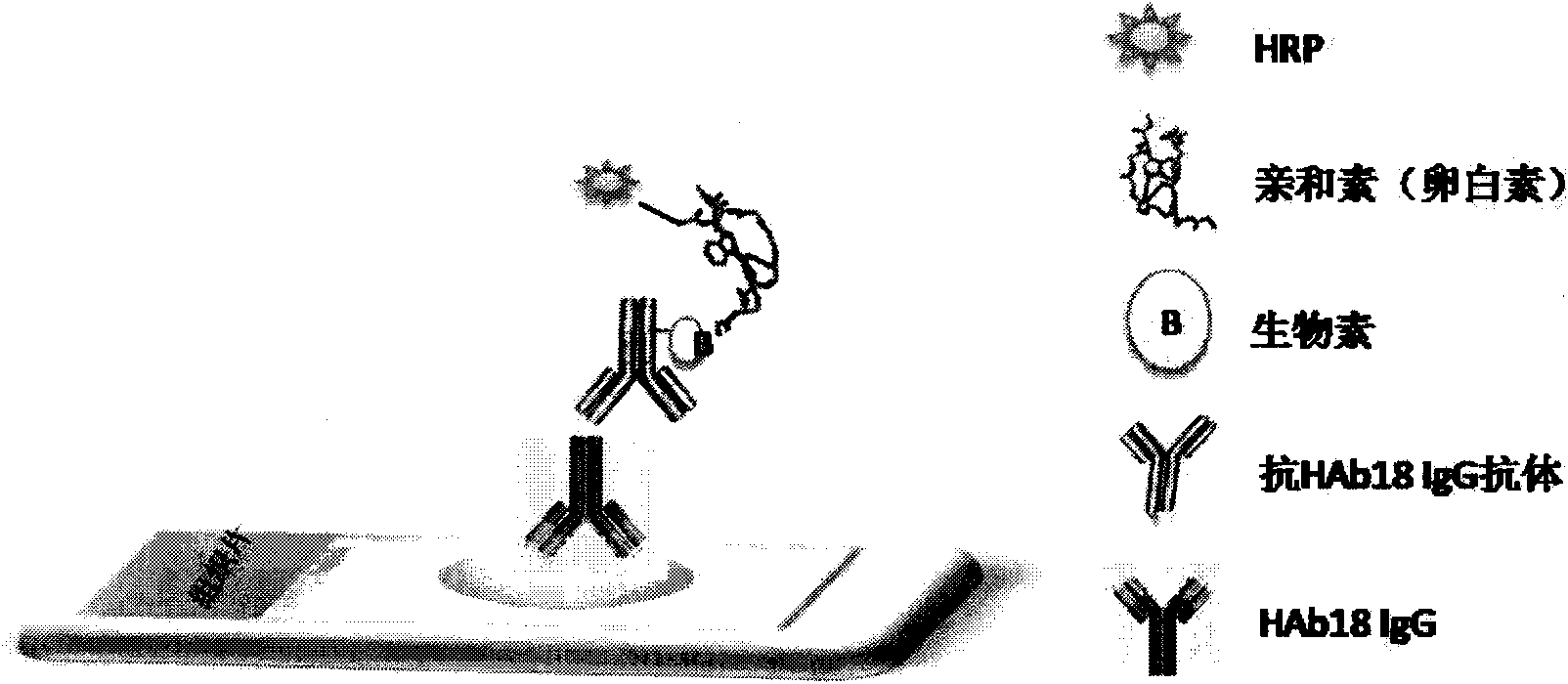Immune tissue chemical diagnostic kit used for pathological diagnosis of tumour
An immunohistochemistry and diagnostic kit technology, applied in the field of bioengineering, which can solve the problems of poor repeatability, unsatisfactory results, and large differences between antibody batches
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment
[0047] The experimental method of the HAb18G / CD147 cancer marker immunohistochemical diagnostic kit is as follows, and the following examples refer to this method, referred to as "experimental method" for short:
[0048] 1. Sections of paraffin specimens of clinical tissue cases were dewaxed in xylene for 20 minutes, three times;
[0049] 2. 100% alcohol to remove xylene, 5min, twice; 95%-75% alcohol, 2min / time;
[0050] 3. 3% H2O2 to block endogenous peroxidase, 22-28°C, 10 minutes;
[0051] 4. Soak in PBS buffer for 3 minutes, 3 times;
[0052] 5. 5% normal sheep serum to block non-specific sites in the tissue, 22-28°C, 20 minutes;
[0053] 6. HAb18 monoclonal antibody (30ug / mL), 37°C, 120 minutes;
[0054] 7. Soak in PBS buffer for 5 minutes, 3 times;
[0055] 8. Avidin-labeled anti-specific anti-antibody, 22-28°C, 15 minutes;
[0056] 9. Soak in PBS buffer for 5 minutes, 3 times;
[0057] 10. Horseradish peroxidase-labeled biotin, 22-28°C, 20 minutes;
[0058] 11. S...
Embodiment 1
[0065] The HAb18G / CD147 cancer marker immunohistochemical diagnostic kit for auxiliary diagnosis or differential diagnosis of cancer is expressed in liver tissue. The specific test process is as follows:
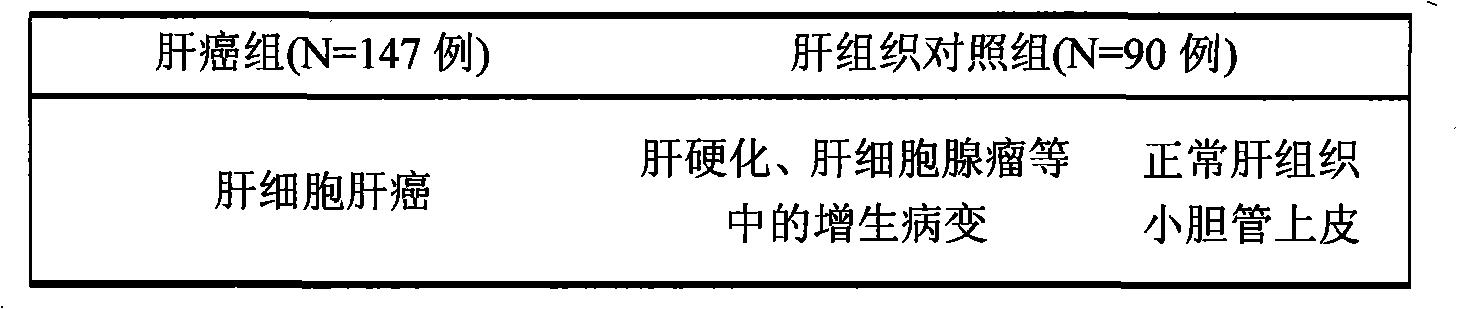
[0066] 1. Sample selection: (liver cancer, cases determined according to the gold standard HE staining)
[0067] Table 1 Case selection of liver cancer and liver tissue
[0068]
[0069] 2. Test operation: see "Experimental Method" for details.
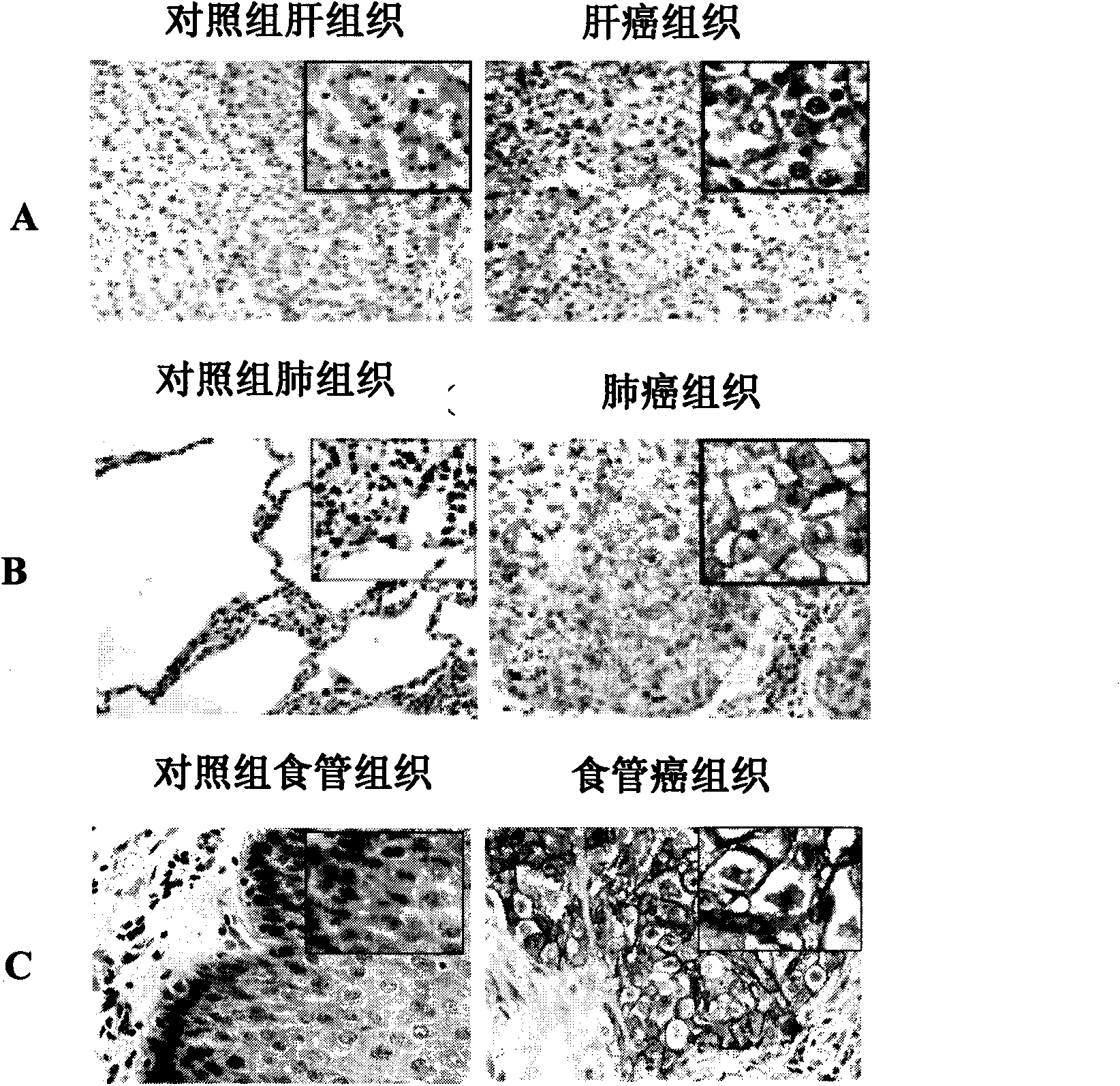
[0070] 3. Test results: (see attached figure 2 A)
[0071] Table 2 Staining results of HAb18G / CD147 cancer marker diagnostic kit in liver cancer and liver tissue
[0072]
Embodiment 2
[0074] HAb18G / CD147 cancer marker immunohistochemical diagnostic kit for auxiliary diagnosis or differential diagnosis of cancer expressed in lung tissue The specific test process is as follows:
[0075] 1. Sample selection: lung cancer, according to the results of the gold standard HE staining method to determine the case
[0076] Table 3: Case selection for lung cancer and lung tissue
[0077]
[0078] 2. Test operation: see "Experimental Method" for details.
[0079] 3. Test results: (see attached figure 2 B)
[0080] Table 4: Staining results of HAb18G / CD147 cancer marker diagnostic kit in lung cancer and lung tissue
[0081]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com