Method for removing sulphur from coking benzol
A technology for coking benzene and oxidant, applied in chemical instruments and methods, molecular sieve catalysts, production of bulk chemicals, etc., can solve the problems of high cost, large amount of three wastes, high corrosion of equipment, etc., achieving less three wastes and simple process flow , The effect of high desulphurization efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
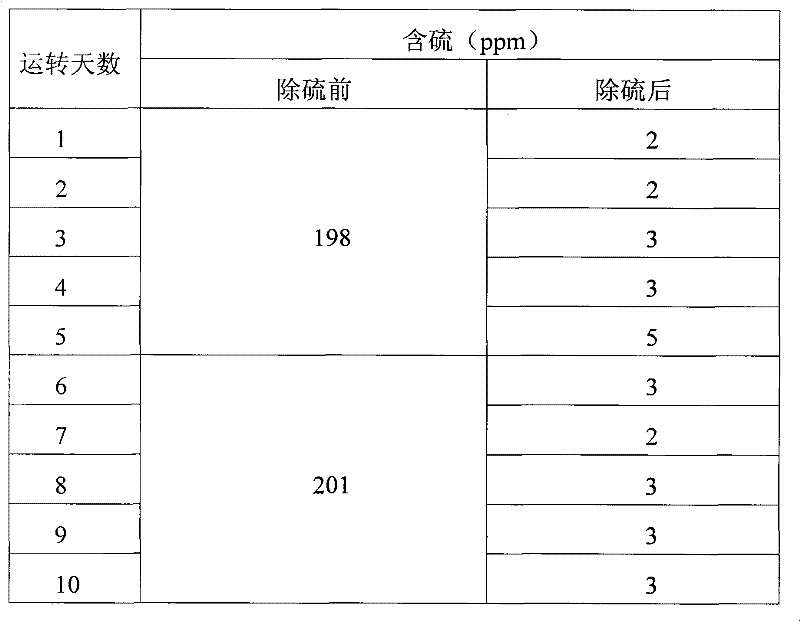
Embodiment 1
[0027] Three kettles connected in series, each kettle volume: 20L, 20L, 20L, the total effective reaction volume is 30L. The reaction temperature is 55°C to 65°C, and the exothermic heat of the reaction is exchanged through the jacket. Catalyst Titanium silicon molecular sieve catalyst fine particles (particle size 1-3um) account for 5% of the weight of coked benzene. Methanol is 50% of the weight of coked benzene, hydrogen peroxide is 5% of the weight of coked benzene, and the content is 27.5% (volume percentage).
[0028] Firstly, after the clinker is layered according to the proportion gap reaction, the slurry is put into the high-level tank, and the coking benzene, catalyst slurry and H 2 o 2 Use a peristaltic pump to drive into the first-stage reaction kettle at the same time, and the flow rates are V BZ =7.1L / hr, V H2O2 =56mL / hr, slurry V=2.9L / hr, add oxidant hydrogen peroxide dropwise in the first-stage reactor or each reactor. The stirring speed is 350rpm, the thr...
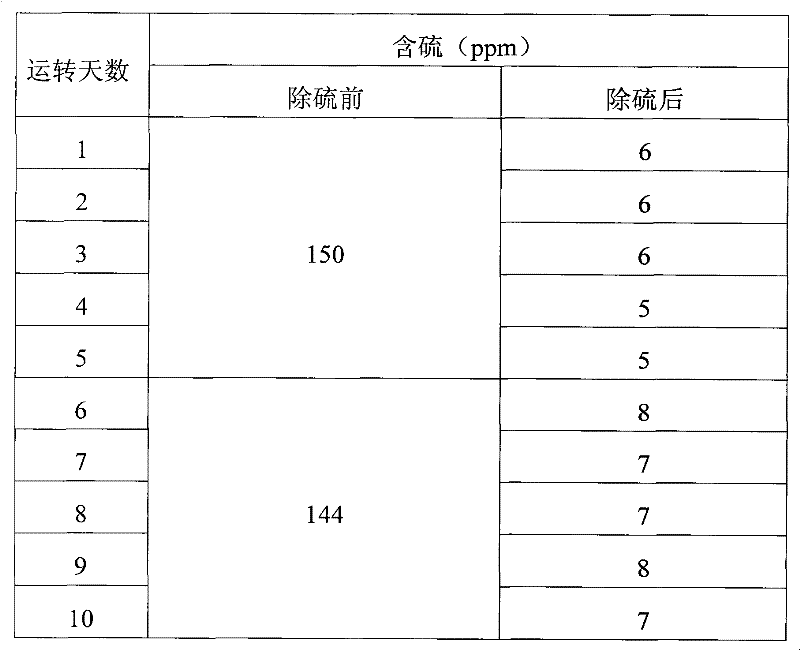
Embodiment 2
[0032] Three kettles connected in series, the volume of each kettle: 20L, 20L, 20L, the effective reaction volume is 30L, the reaction temperature is 30°C-40°C, and the catalyst is 5% of the weight of coked benzene. Methanol is 50% of the weight of coked benzene, and the content of hydrogen peroxide is 35% (percentage by volume). The consumption is 5% of the weight of coked benzene.
[0033] Firstly, after the clinker is layered according to the proportion gap reaction, the slurry is put into the high-level tank, and the coking benzene, catalyst slurry and H 2 o 2 Use a peristaltic pump to drive into the first-stage reaction kettle at the same time, and the flow rates are V BZ =7.1L / hr, V H2O2 =56mL / hr, slurry V=2.9L / hr, add oxidant hydrogen peroxide dropwise in the first-stage reactor or each reactor. The stirring speed is 350rpm, the three reactors stay for about 3 hours, the feed flow rate per hour = 10L / h, and the reaction materials overflow from the upper end of the re...
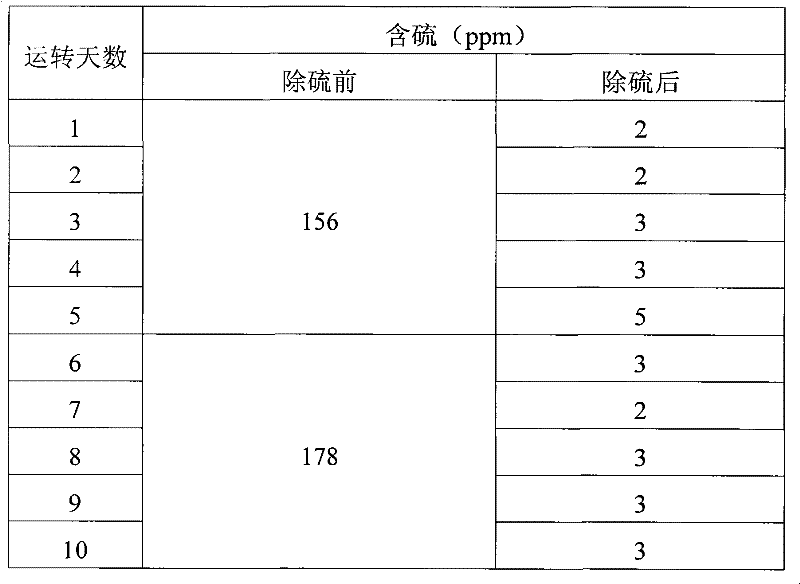
Embodiment 3
[0036] Three kettles connected in series, each kettle volume: 20L, 20L, 20L, the total effective reaction volume is 30L. The reaction temperature is 55°C-65°C, the reaction is exothermic, and the heat is exchanged through the jacket. The catalyst is 5% by weight of coked benzene. Methanol is 20% of the weight of coked benzene, and the content of hydrogen peroxide is 27.5% (volume percentage). The consumption is 5% of the weight of coked benzene.
[0037] Firstly, after the clinker is layered according to the proportion gap reaction, the slurry is put into the high-level tank, and the coking benzene, catalyst slurry and H 2 o 2 Use a peristaltic pump to drive into the first-stage reaction kettle at the same time, and the flow rates are V BZ =7.1L / hr, V H2O2 =56mL / hr, slurry V=2.9L / hr, add oxidant hydrogen peroxide dropwise in the first-stage reactor or each reactor. The stirring speed is 350rpm, the three reactors stay for about 3 hours, the feed flow rate per hour = 10L / h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| percent by volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com