Halogenated hydroxyl aromatic methane compounds, preparation method and use thereof
A technology for halogenated hydroxyaromatic methanes and compounds, applied in the field of new compounds and their preparation, can solve the problems of low yield, difficult research and high extraction cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
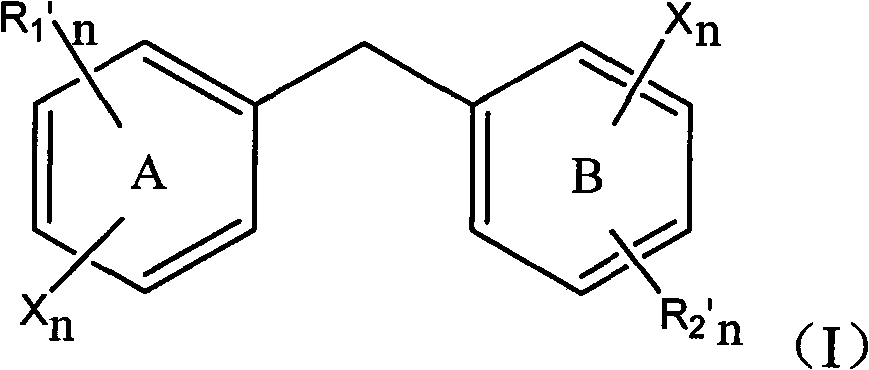
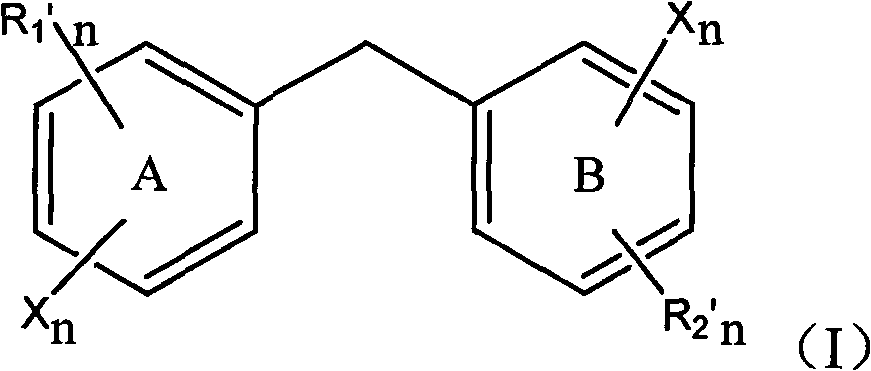
[0067] Embodiment 1: 2,3-dibromo-4, the preparation of 5-dihydroxydiphenylmethane I
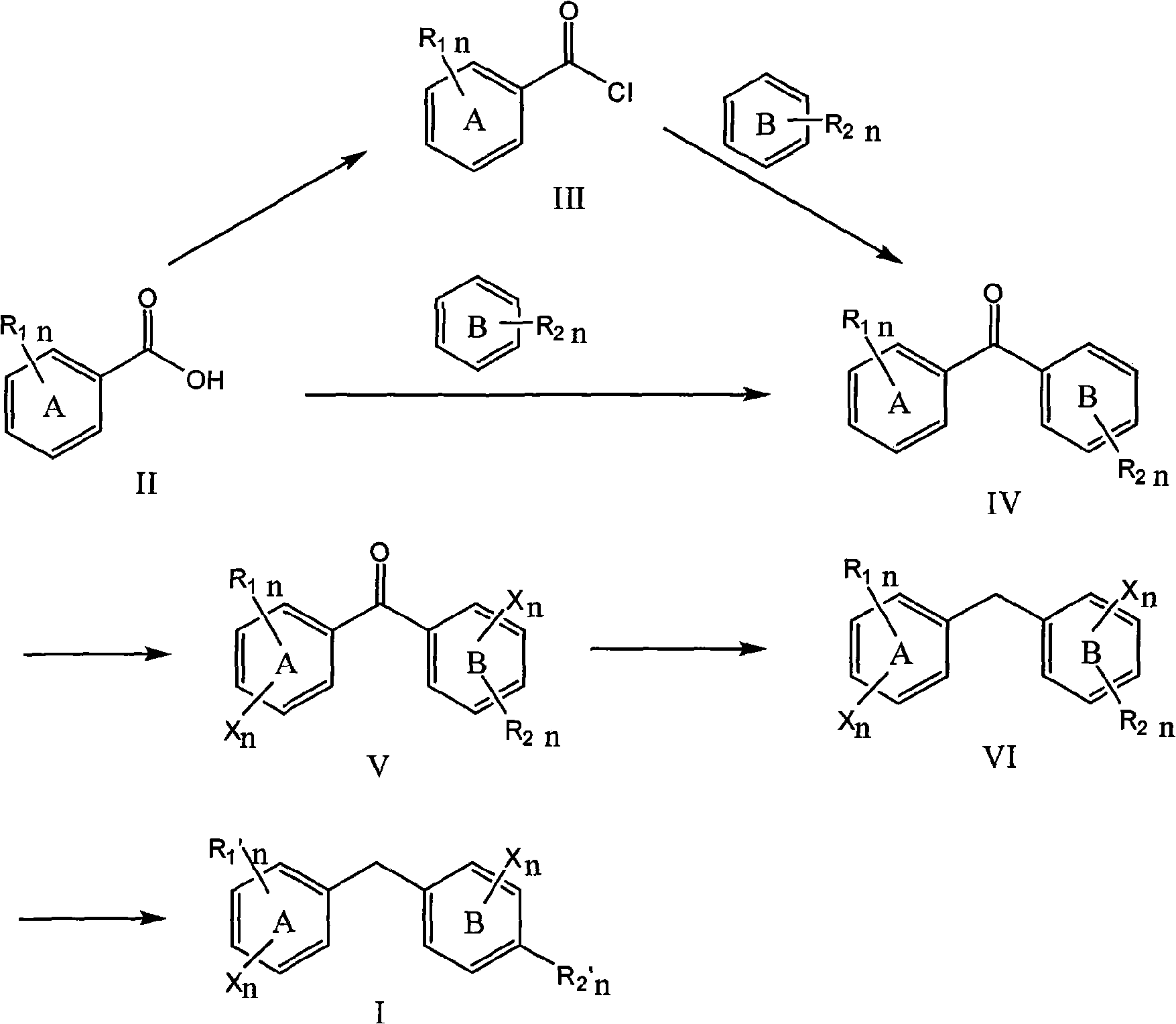
[0068] (1) 3, the preparation of 4-dimethoxybenzophenone IV
[0069] Put 30ml of anhydrous dichloromethane in an eggplant-shaped bottle, add 5ml (0.036mol) of 1,2-dimethoxybenzene and 5.600g (0.040mol) of benzoyl chloride III under ice-bath conditions, and slowly add trichloromethane in batches Aluminum 5.800g (0.042mol), the system turns brownish red, after 10min, remove the ice and stir in the bathroom, TLC monitors that the reaction is complete after 4h, add ice water to terminate the reaction, extract twice with 50ml dichloromethane, collect the oil phase and dry it. Sodium sulfate was dried and left overnight, and the solvent was removed by rotary evaporation to obtain 7.138 g of a white solid with a melting point of 100°C-102°C and a yield of 82%. 1 HNMR (500MHz, CDCl 3 )δ: 3.984 (d, 6H, CH 3 ), 6.922 (d, 1H, Ph), 7.408 (d, 1H, Ph), 7.508 (t, 3H, Ph), 7.602 (t, 1H, Ph), 7.790 (d, 2H,...
Embodiment 2
[0076]Embodiment 2: 2-chloro-3', the preparation of 4'-dihydroxydiphenylmethane I
[0077] (1) Preparation of 2-chloro-3', 4'-dimethoxybenzophenone V
[0078] Put 30ml of anhydrous dichloromethane in an eggplant-shaped bottle, add 1,2-dimethoxybenzene 5ml (36mmol) at 20 degrees Celsius, p-chlorobenzoyl chloride III 5ml (40mmol), slowly add aluminum trichloride 5.800g ( 42mmol), the system turned brownish red, after 10 minutes, stirred at room temperature, TLC monitored the reaction completely after 1h, added ice water to terminate the reaction, extracted twice with 50ml dichloromethane, collected the oil phase, dried with anhydrous sodium sulfate and placed overnight, The solvent was removed by rotary evaporation to obtain 9.832 g of a white solid with a melting point of 137° C. to 139° C. and a yield of 92%. 1 HNMR (500MHz, CDCl 3 )δ: 3.98 (d, 6H, CH 3 ), 6.87 (d, 1H, Ph), 7.24 (d, 1H, Ph), 7.39 (d, 2H, Ph), 7.43-7.51 (m, 2H, Ph), 7.62 (s, 1H, Ph). ESI-MS (m / z): 277.1, 27...
Embodiment 3
[0083] Embodiment 3: 2,6-dibromo-3,3',4,4', the preparation of 5-trihydroxydiphenylmethane I
[0084] (1) 3,3',4,4', the preparation of 5-pentamethoxybenzophenone IV
[0085] Add 5ml (36mmol) of 1,2-dimethoxybenzene and 20ml of polyphosphoric acid into the eggplant-shaped bottle, stir and dissolve, then add 7.632 (36mmol) of 3,4,5-trimethoxybenzoic acid, and react in an oil bath at 150°C , TLC monitored the reaction to be complete after 4 hours. The reaction was quenched with ice water, and 100 ml of distilled water was added to precipitate a gray solid, which was recrystallized from methanol to obtain 6.520 g of a white solid with a melting point of 115° C. to 117° C. and a yield of 88%. 1 HNMR (500MHz, CDCl 3 )δ: 3.908(s, 6H, CH 3 ), 3.977(t, 9H, CH 3 ) 6.935 (d, 1H, Ph), 7.052 (s, 2H, Ph), 7.430 (d, 1H, Ph), 7.487 (s, 1H, Ph). ESI-MS (m / z): 333.2 ([M+H] + ), 355.2 ([M+Na] + ).
[0086] (2) Preparation of 2,6-dibromo-3,3',4,4',5-pentamethoxybenzophenone V
[0087] We...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com