Preparation and application of chlamys farreri peptidoglycan recognition protein (Cf-PGRP) with sterilizing activity
A technique for identifying proteins, Chlamys farreri, applied in the field of molecular biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0020] The in vitro prokaryotic recombinant expression of the Chlamys farreri peptidoglycan recognition protein Cf-PGRP gene coding region comprises the following steps:
[0021] 1. Construction of recombinant vector
[0022] The recombinant vector used in the present invention is the pET prokaryotic expression system of Novagen Company.
[0023] By PCR technique, gene-specific primers P1 (5'-GGATCCATTACAGCTGATTACCTGATAAC-3') and P2 (5'-GCGGCCGCTTAAGGGCATCCCGGAC-3') with specific restriction sites of BamH I and Not I added at the 5' end were used to amplify the comb A fragment of the coding region of the peptidoglycan recognition protein Cf-PGRP of the scallop scallop. The reaction conditions were as follows: 94°C pre-denaturation for 5 minutes, and then the following cycles: 94°C denaturation for 30 seconds, 60°C annealing for 30 seconds, 72°C extension for 30 seconds, a total of 30 cycles, and finally 72°C extension for 10 minutes. The amplified fragment was purified and r...
Embodiment 2
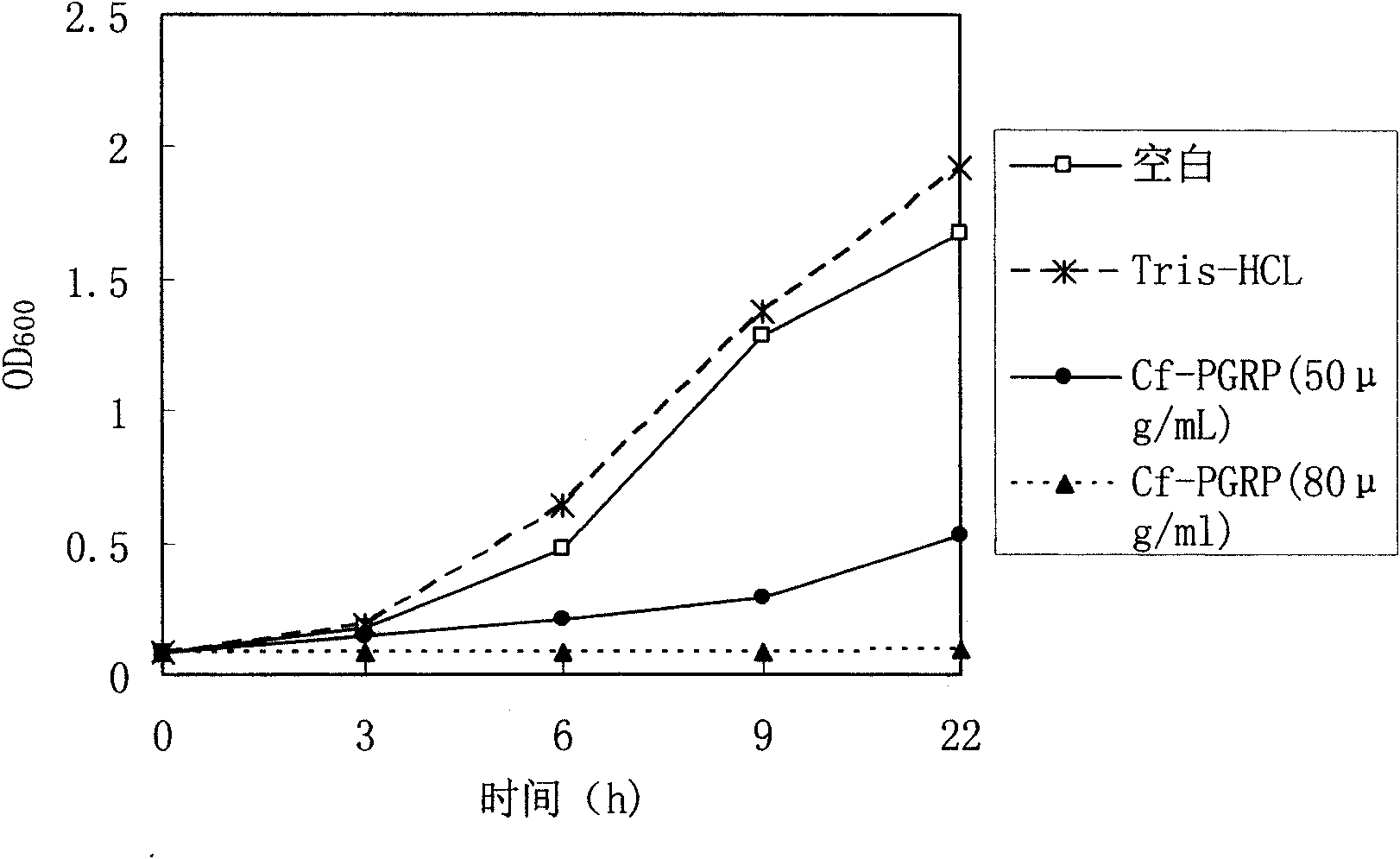
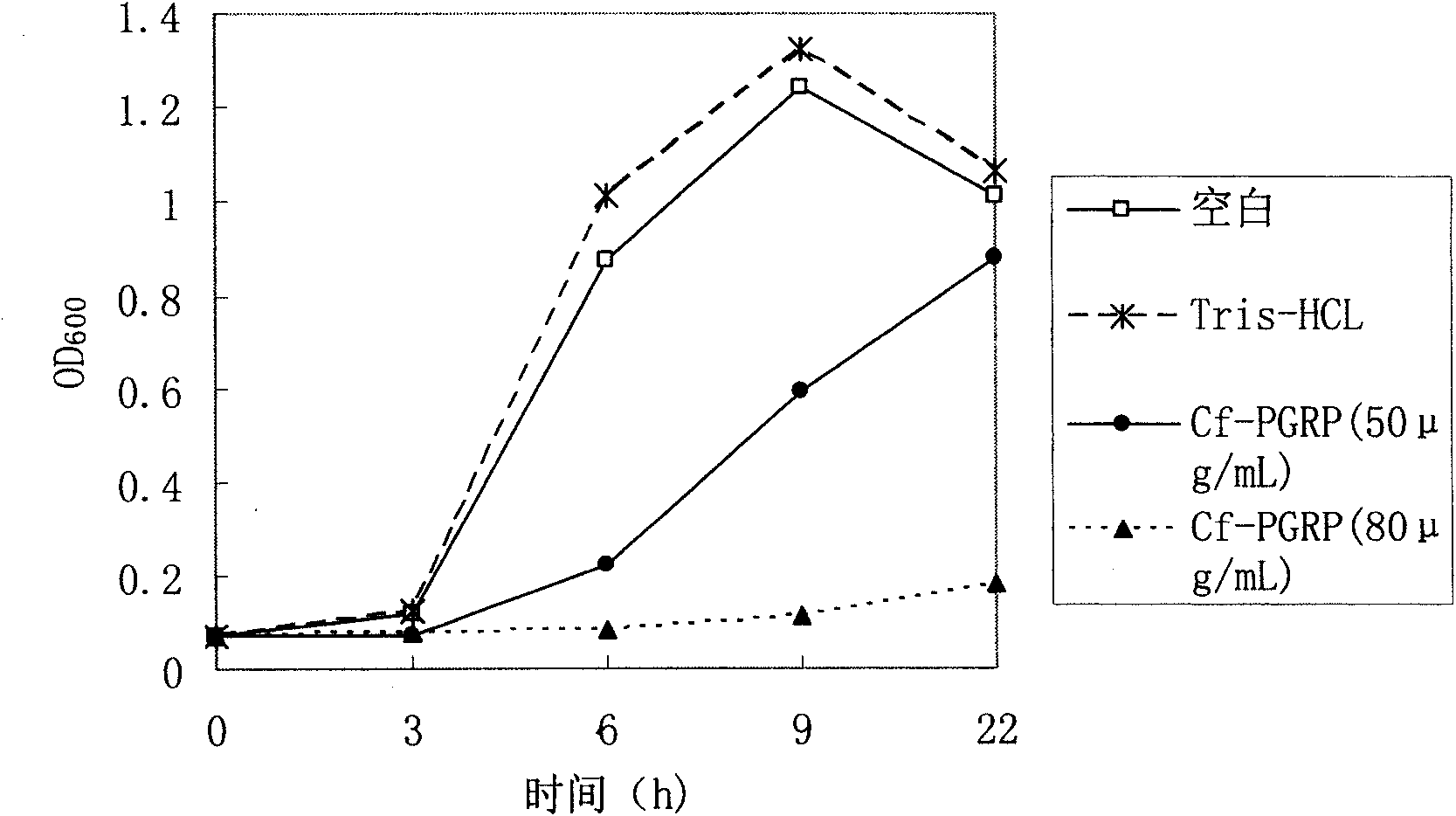
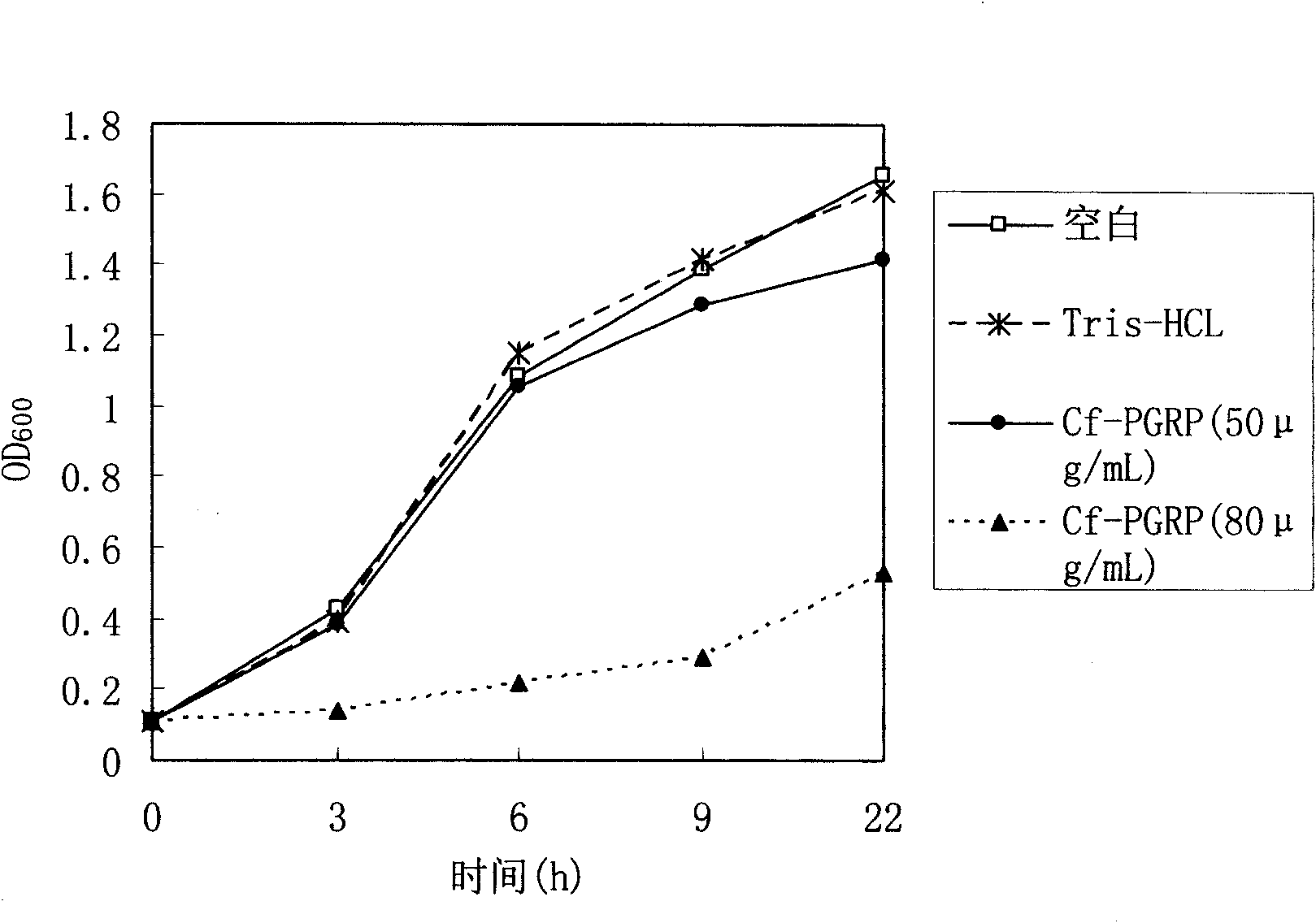
[0031] Low concentration of Cf-PGRP protein can inhibit the reproduction of Gram-positive bacteria, while high concentration can kill it. This protein also has a certain antibacterial effect on some Gram-negative bacteria. It has potential applications in the development of broad-spectrum antibacterial drugs, immune enhancers and feed additives.
[0032] Growth inhibition experiment on Micrococcus luteus, Bacillus subtilis and Escherichia coli: Take 36 sterile test tubes and arrange them in three rows, add 1mL of SOB liquid medium and 10μL of logarithmic growth phase bacterial suspension to each tube, and then add to the test tube Add recombinant protein and Tris-HCl control solution with a final concentration of 50 μg / mL and 80 μg / mL, incubate at 220 rpm at 37°C, and observe OD at 0h, 3h, 6h, 9h, and 22h 600 Three repetitions were set up for each sample, and the average value was plotted according to the three measured results. Experiments have shown that low concentration ...
Embodiment 3
[0033] Implementation Example 3: Determination of the minimum inhibitory concentrations of Micrococcus luteus, Bacillus subtilis and Escherichia coli.
[0034] Bacteria were cultured at 37°C to OD600=0.5, diluted 1×10 4 Incubate with the recombinant protein and Tris-HCl control solution with a final concentration range of 0 μg / mL, 1 μg / mL, 2 μg / mL, 4 μg / mL, 8 μg / mL, 16 μg / mL, 32 μg / mL, 64 μg / mL for 30 min, Pour into the SOB plate for culture, and after the plate is cultured at room temperature for 18 hours, the minimum protein concentration that does not allow any visible colony growth is the minimum inhibitory concentration (MIC). Three replicates are set for each sample. Results: The minimum inhibitory concentrations of the recombinant protein against Micrococcus luteus, Bacillus subtilis and Escherichia coli were 2 μg / mL, 2 μg / mL and 4 μg / mL, respectively.
Description of drawings:
[0035] The figure shows the inhibitory effect of recombinant peptidoglycan recognition pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com