Glipizide sustained-release capsule formulation and preparation method thereof
A technology of glipizide and sustained-release capsules, applied in the field of glipizide sustained-release capsules and its preparation, can solve problems such as hypoglycemia and insufficient blood sugar control, achieve good blood sugar levels, good therapeutic effects, and reduce blood sugar fluctuations Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: Preparation of glipizide sustained-release pellets (Glipizide No. 1 pellets)
[0047] formula:
[0048] Glipizide 10g
[0049] Beta Cyclodextrin 3-6g
[0050] Starch 140g
[0051] NaCl fine powder 40g
[0052] Dextrin 20g
[0053] Ethyl cellulose 1.1~4.4g
[0054] Polyethylene glycol 6000 0.55~2.2g
[0055] Appropriate amount of 7.3% PVPK30 ethanol solution (50%)
[0056] Preparation Process:
[0057] Prepare blank pellets→prepare drug-containing pellets→prepare sustained-release pellets
[0058] Preparation:
[0059] I. Preparation of blank pellets
[0060] Fully mix 140g of starch, 20g of dextrin and 40g of sodium chloride fine powder, pass through an 80-mesh sieve, put in a coating granulator, and use 7.3% PVPK30 ethanol solution (50%) as a binder to make pellets. When spheronized to 28-20 mesh, take it out and dry at 60°C, select 28-20 mesh blank pellets, and set aside;
[0061] II. Preparation of drug-containing pellets
[0062] Get 10g of ...
Embodiment 2
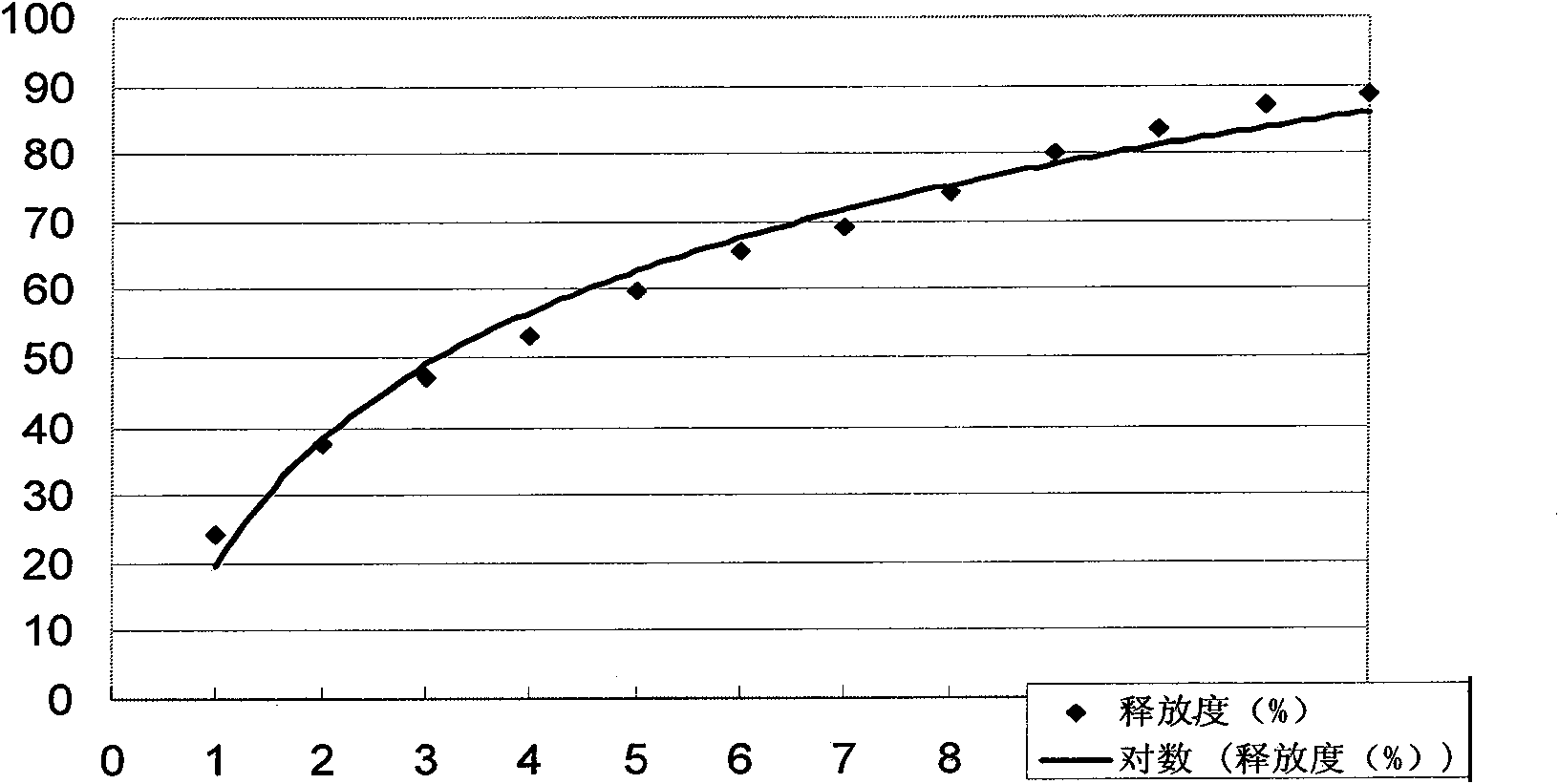
[0067] Example 2 Determination of the release rate of glipizide sustained-release pellets
[0068] Get the embodiment 1 product of the present invention, according to the release assay method (Chinese Pharmacopoeia version two appendix XD first method in 1995), adopt the second method device of dissolution assay, be solvent with the artificial intestinal fluid 900ml not containing pancreatin, rotating speed is 50 rpm, operate according to the law, every 1 hour, take 8ml of the solution respectively, filter, take the filtrate as the test solution, and immediately add the same temperature and the same amount of the above solvent to the dissolution cup. Use high-performance liquid chromatography (model LC-10A, produced by Shimadzu Corporation of Japan) to measure release in Table 1:
[0069] Table 1 Example 1 of the present invention Glipizide sustained-release pellet release-time data table
[0070] time
[0071] Its release curve is shown in figure 1 .
Embodiment 3
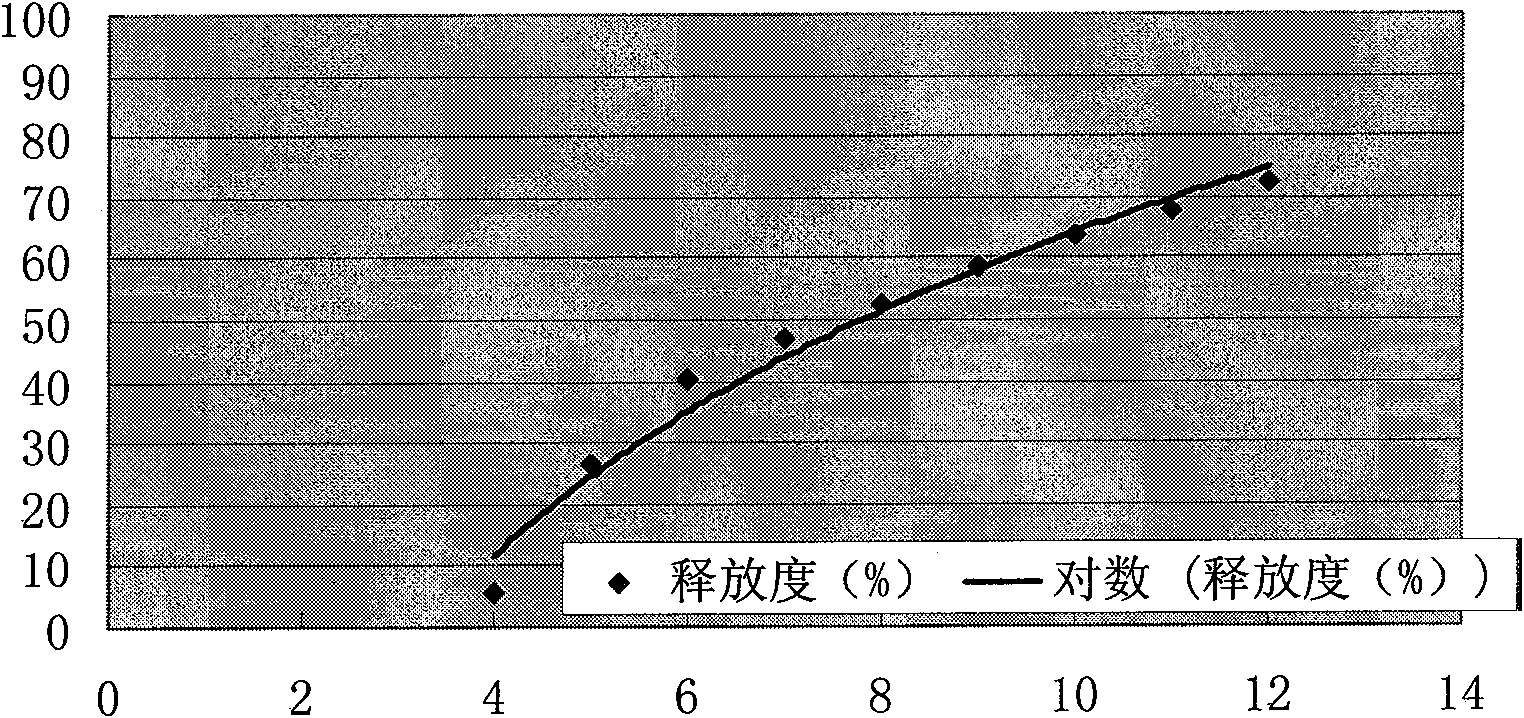
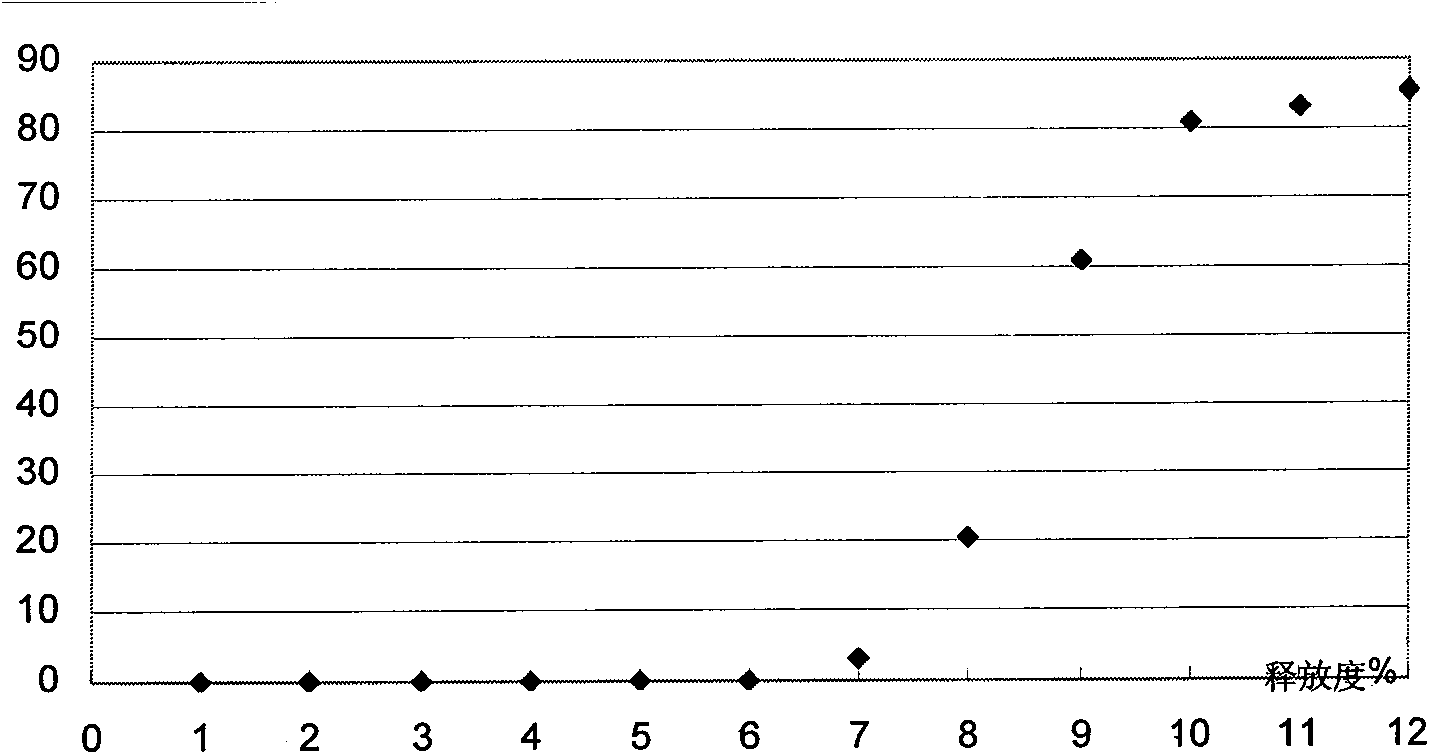
[0072] Example 3 Preparation of Glipizide 4-hour Delayed-release Sustained-release Pellets (Glipizide No. 2 Pellets)
[0073] formula:
[0074] Glipizide Extended Release Pellets 200g
[0075] Ethyl cellulose 1-3g
[0076] Preparation:
[0077] 1. Get water-insoluble material ethyl cellulose (EC) 1g, dissolve with 10ml ethanol and make coating solution, set aside.
[0078] II. Put 80 g of the glipizide sustained-release pellets prepared according to Example 1 in a coating machine, and use an atomizing spray gun to spray the above-mentioned coating solution onto the glipizide pellets: adjust the nozzle pressure to make the coating solution spray To achieve the best atomization effect, blow in hot air, adjust the speed to achieve the best turning effect, spray into the coating solution, take it out and ventilate and dry at 60°C, select 30-20 mesh pellets and mix well to get glipizide 4 The sustained-release pellets are hourly delayed release, and the thickness of the coating...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com