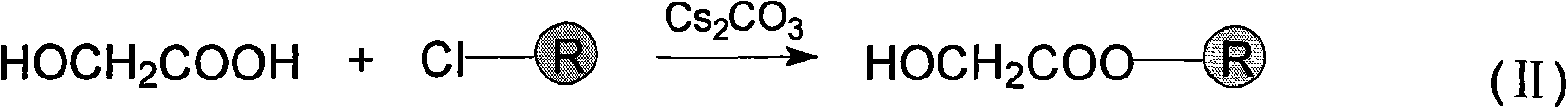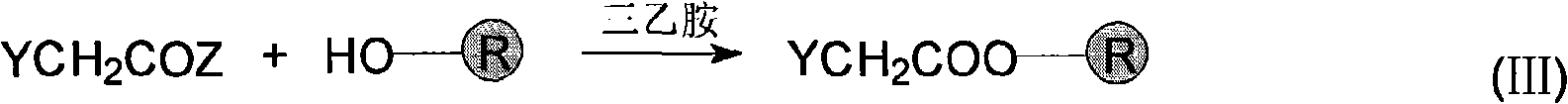New method for solid-phase synthesis of side-chain-protected peptide chain
A synthesis method and peptide chain technology, which is applied in the field of solid-phase synthesis and preparation of organic compounds and peptides, can solve the problems of high price, limited application, high price, etc., and achieve the effect of increasing synthesis cost and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0022] (1) Connection between "handle" and Merrifield resin - glycolic acid reacts with Merrifield resin
[0023]
[0024] Take by weighing 120mg (0.08mmol) Merrifield resin (0.67mmol / g) and join in the solid phase reactor, add 4.0ml dichloromethane, after shaking on the shaker for 30min, remove dichloromethane under reduced pressure, wash resin 3 with 4.0mlDMF Second-rate. Mix and react 30mg (0.4mmol) glycolic acid and 65mg (0.2mmol) cesium carbonate in 4.0ml DMF for 10min, add the reaction mixture to the solid-phase reactor and mix with the resin, shake it on the shaker for 4 hours, "handle ” attached to the resin via an ester bond. The solvent was removed by filtration, and the resin was first washed twice with 3.0 ml of water to remove cesium chloride generated during the reaction, and then washed four times with 4.0 ml of DMF for the following peptide chain synthesis.
[0025] (2) Synthesize the polypeptide H2N-Ala-Ser(tBu)-Gly-OH on the "handle" in (1)
[0026] ①Co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com