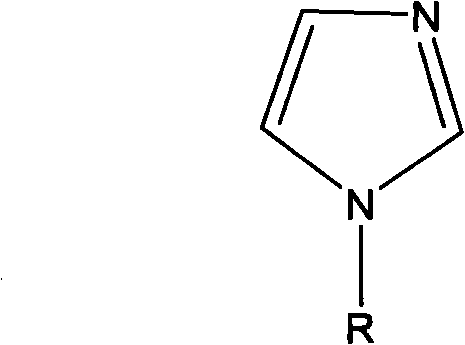
Preparation method of N-alkyl imidazole
A technology for alkyl imidazole and imidazole, applied in the field of synthesizing N-alkyl imidazole, can solve problems such as large energy consumption, and achieve the effect of reducing consumption cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
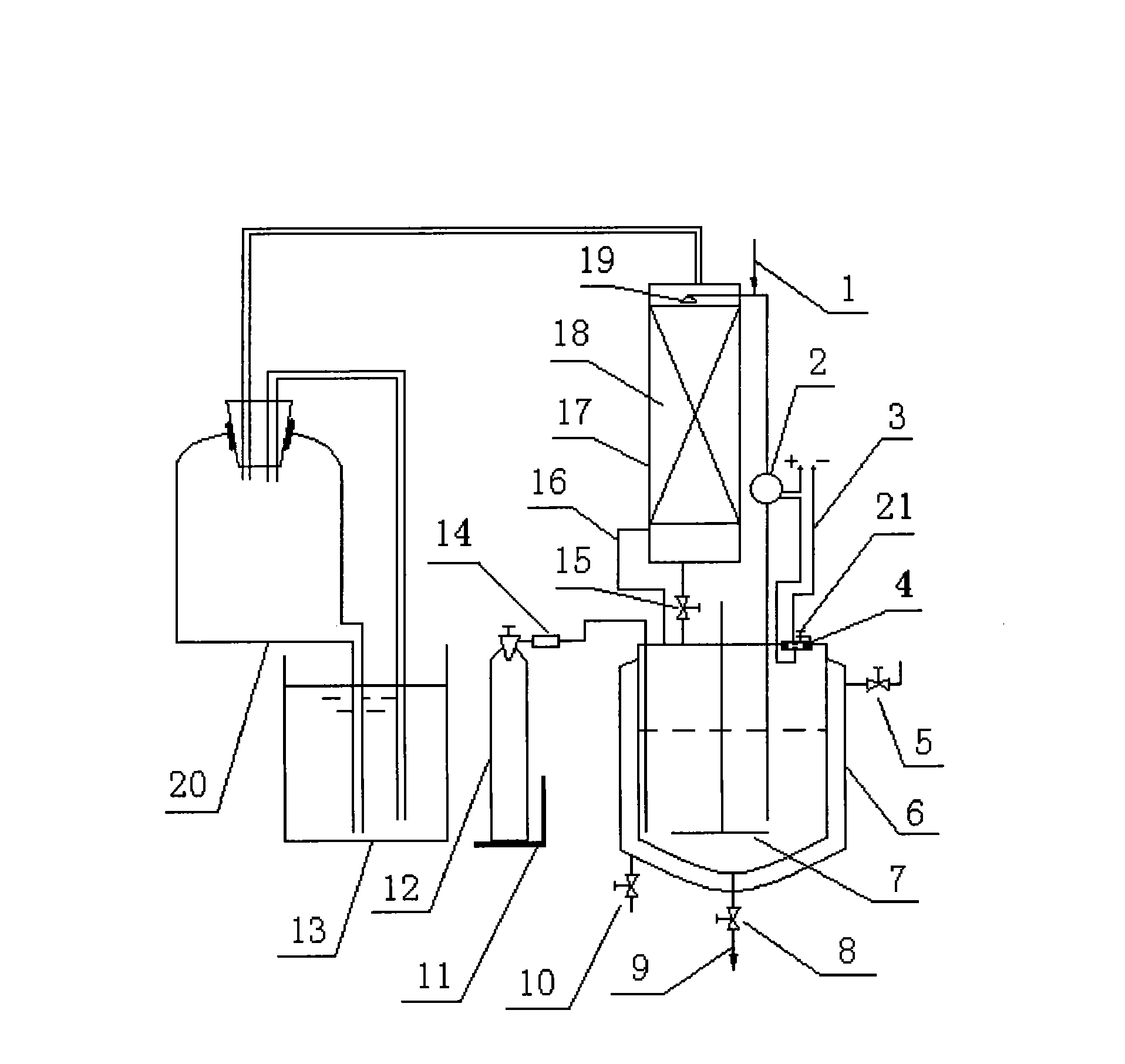
[0019] Embodiment 1: Add 69.3 kilograms (500mol) of 41.84% glyoxal 69.3 kilograms (500mol) and 37.2% formaldehyde 40.3 kilograms (500mol) mixed solution in the reactor 6 of gas-liquid phase synthesis device, open heating steam valve 5 to reactor 6 Carry out heating to 50 ℃, under the situation that stirring blade 7 rotates, open ammonia gas cylinder 12, pass into ammonia gas 21 kilograms (750mol) slowly under micro-negative pressure condition, make liquid-sealed pool 13 almost not have ammonia bubble to emit, according to Measuring scale 11 shows that the data can accurately grasp the amount of ammonia gas charging and discharging, while 55.4 kilograms of methylamine (500mol) aqueous solution of 28% is added dropwise. When the value is fixed, the pump 2 starts to work, and in the absorption tower 17, the ammonia contacts with the sprayed reaction droplets, the ammonia absorption speed is accelerated, and the ammonia pressure drops rapidly. When the ammonia pressure drops below...
Embodiment 2
[0020] Embodiment 2: inject the mixed solution of 69.3 kilograms (500mol) of 41.84% glyoxal and 40.3 kilograms (500mol) of formaldehyde of 37.2% in the reactor of gas-liquid phase synthesis device. Under stirring and controlling slight negative pressure, heat to 50°C, continuously and slowly pass 42 kg (1500mol) of ammonia gas into the mixed aldehyde, and control the temperature at about 55°C. On access to NH 3 During the process, the color of the solution changed from colorless to light yellow, then to golden yellow, orange and finally to brown. Pass through NH 3 After that, it was incubated at 54-55°C for 4h. After the reaction was completed, the water was evaporated, and the fraction at 250°C was collected.
Embodiment 3
[0021] Embodiment 3: Add 69.3 kilograms (500mol) of 41.84% glyoxal and 40.3 kilograms (500mol) of formaldehyde mixed solution of 41.84% in the reactor of gas-liquid phase synthesis device, at 50 ℃, stir and control slight negative pressure Slowly feed 21 kilograms (750mol) of ammonia gas, and simultaneously add 76 kilograms (500mol) of 30% ethylamine solution dropwise; after the addition, continue to control the temperature at 50°C and react for 6 hours; The water was removed and the fraction at 150°C was collected.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com