Respiratory-tract magnetic-resonance spray contrast agent and preparation method thereof
A contrast agent and magnetic resonance technology, which is applied in the direction of nuclear magnetic resonance/magnetic resonance imaging contrast agent, emulsion delivery, drug delivery, etc., can solve the problem of unsatisfactory display effect of gadolinium contrast agent, and is suitable for large-scale preparation and improvement. Sensitivity and specificity, easy-to-use results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
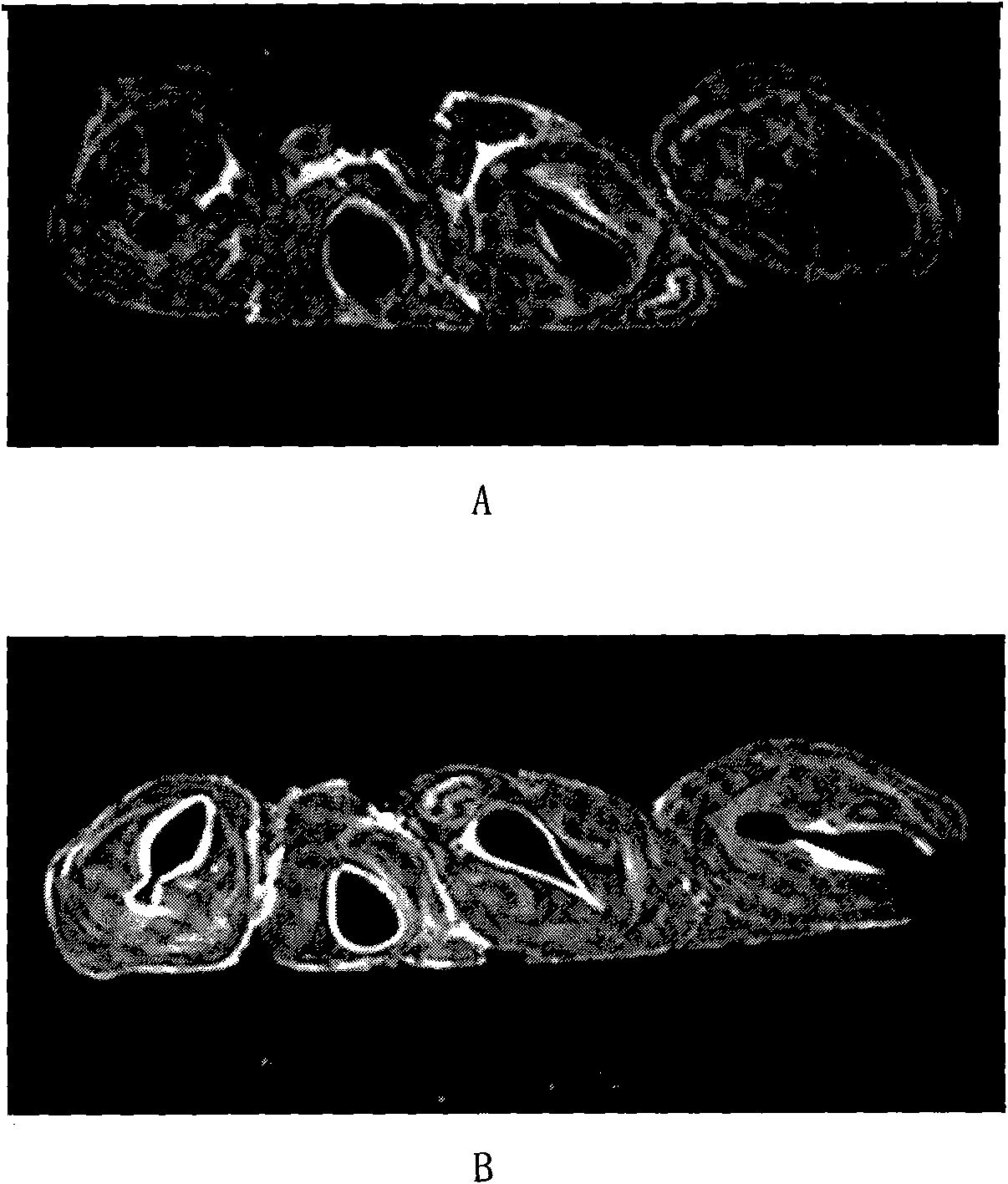
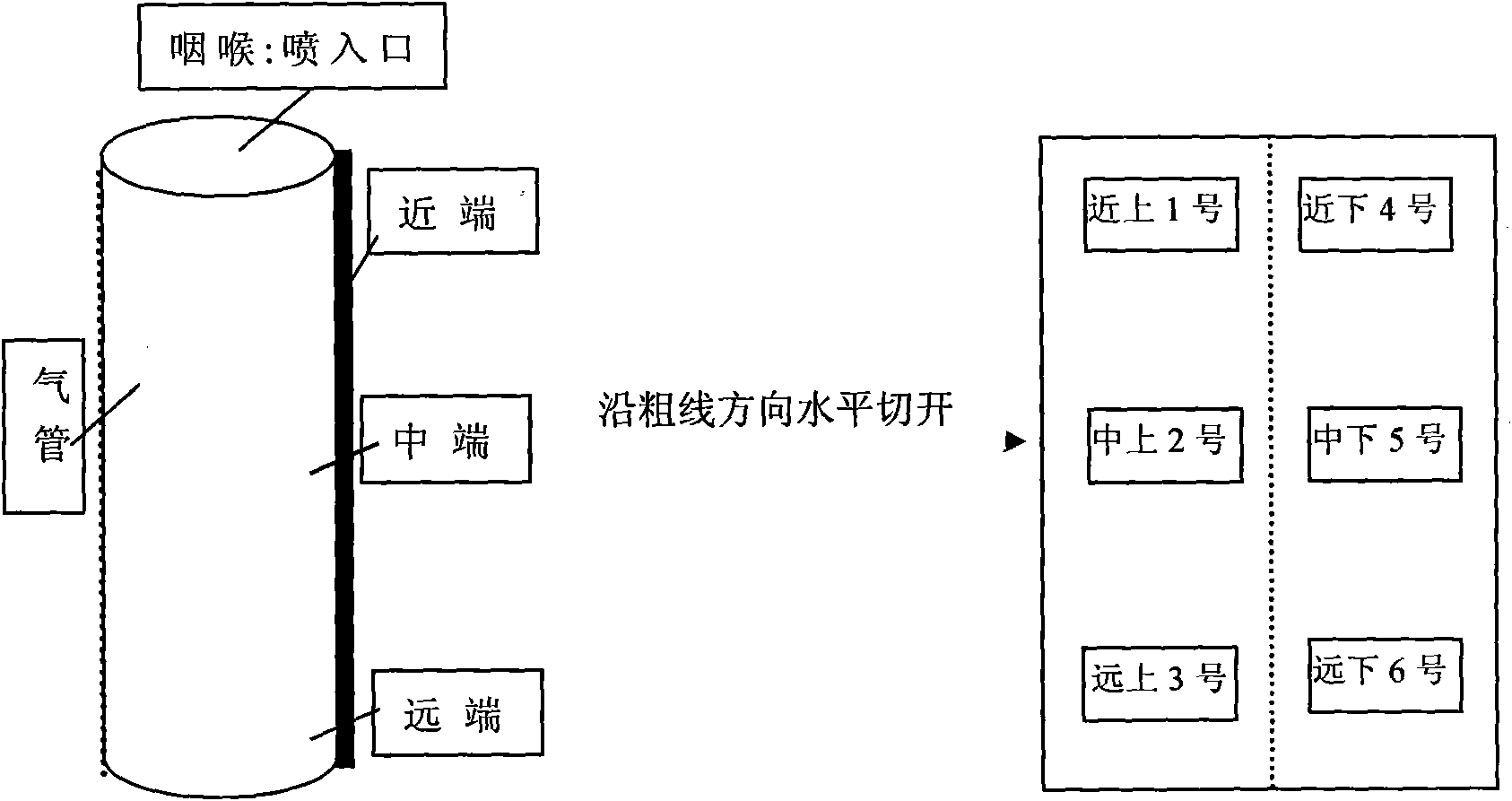
[0027] Gadolinium-lipid complexes were prepared from lecithin, cholesterol and cationic lipid DOTAP (1,2-dioleoyl-3-trimethylammoniopropane). 540 milligrams of lecithin, 180 milligrams of cholesterol and 180 milligrams of DOTAP are dissolved in 10 milliliters of chloroform, and rotary evaporation obtains lipid film, and adding concentration is 30 milliliters of the gadopentetate meglumine aqueous solution of 12 mg / ml (get the gadopentetate meglumine injection Add water to dilute to the required concentration), hydrate, sonicate, and extrude through a 400nm membrane to obtain a gadolinium-lipid complex with a concentration of 12 mg / ml. As determined by PCS, the average particle size of the complex is 400nm, and the zeta potential is 70mv. Ultrasonic nebulization of the obtained compound was carried out to measure the average diameter of the droplets D 50 5um, D 90 It is 8um (Spraytec, British Malvern Company).
[0028] In vitro airway imaging study of gadolinium-lipid comple...
Embodiment 2
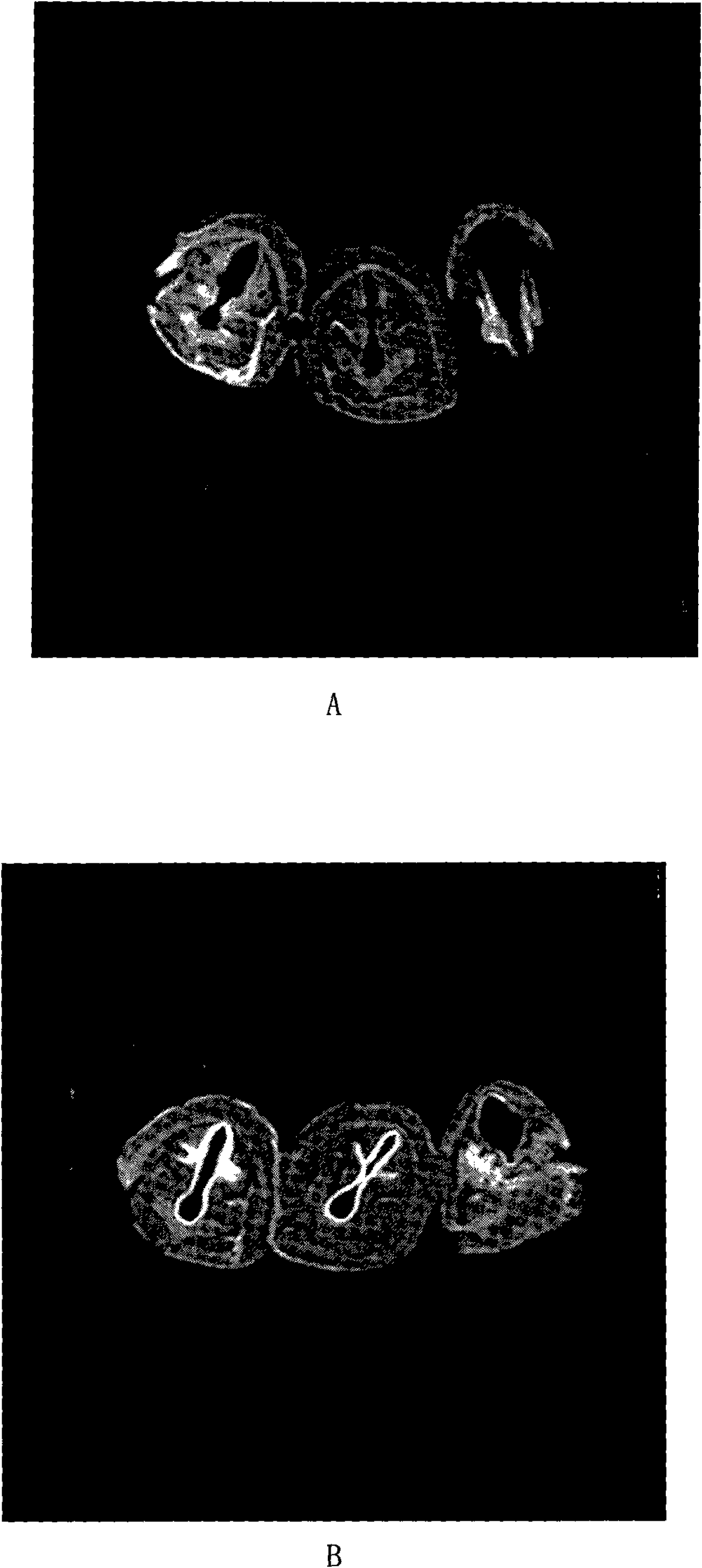
[0031] Gadolinium-lipid complexes were prepared from soybean lecithin, cholesterol and polycationic lipid dioctadecylamidoglycyl spermine (DOGS). 540 milligrams of soybean lecithin, 180 milligrams of cholesterol and 90 milligrams of DOGS are dissolved in 10 milliliters of chloroform, and rotary evaporation obtains lipid film, and adding concentration is 30 milliliters of the aqueous solution of 5 mg / ml of gadobeglumine (get the gadobenate meglumine injection Add water to dilute to the desired concentration), hydrate, sonicate, and homogenize under high pressure to obtain a gadolinium-lipid complex with a concentration of 5 mg / ml. As determined by PCS, the average particle size of the complex is 600nm, and the Zeta potential is 90mv. Ultrasonic nebulization of the obtained compound was carried out to measure the average diameter of the droplets D 50 4um, D 90 It is 9um (Spraytec, British Malvern Company). The results of MRI imaging and gadolinium distribution measurement sho...
Embodiment 3
[0033] Gadolinium-lipid complexes were prepared from DSPC (distearoylphosphatidylcholine), cholesterol and stearylamine. 600 mg of soybean lecithin, 300 mg of cholesterol and 120 mg of stearylamine are dissolved in 20 milliliters of tert-butanol, and 5 ml of concentration of 90 mg / ml of gadopentetate meglumine aqueous solution (dilute gadopentetate meglumine injection with water to desired concentration), and mix well to obtain a clear solution. Freeze-dried for 72 hours, took out and added 30ml of water, hydrated, ultrasonicated, and homogenized under high pressure. A gadolinium-lipid complex was obtained at a concentration of 15 mg / ml. As determined by PCS, the average particle size of the complex is 800nm, and the Zeta potential is 50mv. Ultrasonic nebulization of the obtained compound was carried out to measure the average diameter of the droplets D 50 5.5um, D 90 It is 10um (Spraytec, British Malvern Company). The results of MRI imaging and gadolinium distribution me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com