Device for evaluating dissolving/absorbing process of medical solid preparation
A solid preparation and absorption process technology, which is applied in the field of devices for evaluating the dissolution/absorption process of pharmaceutical solid preparations, and can solve problems such as pipeline blockage, test inability to continue, uneven force, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
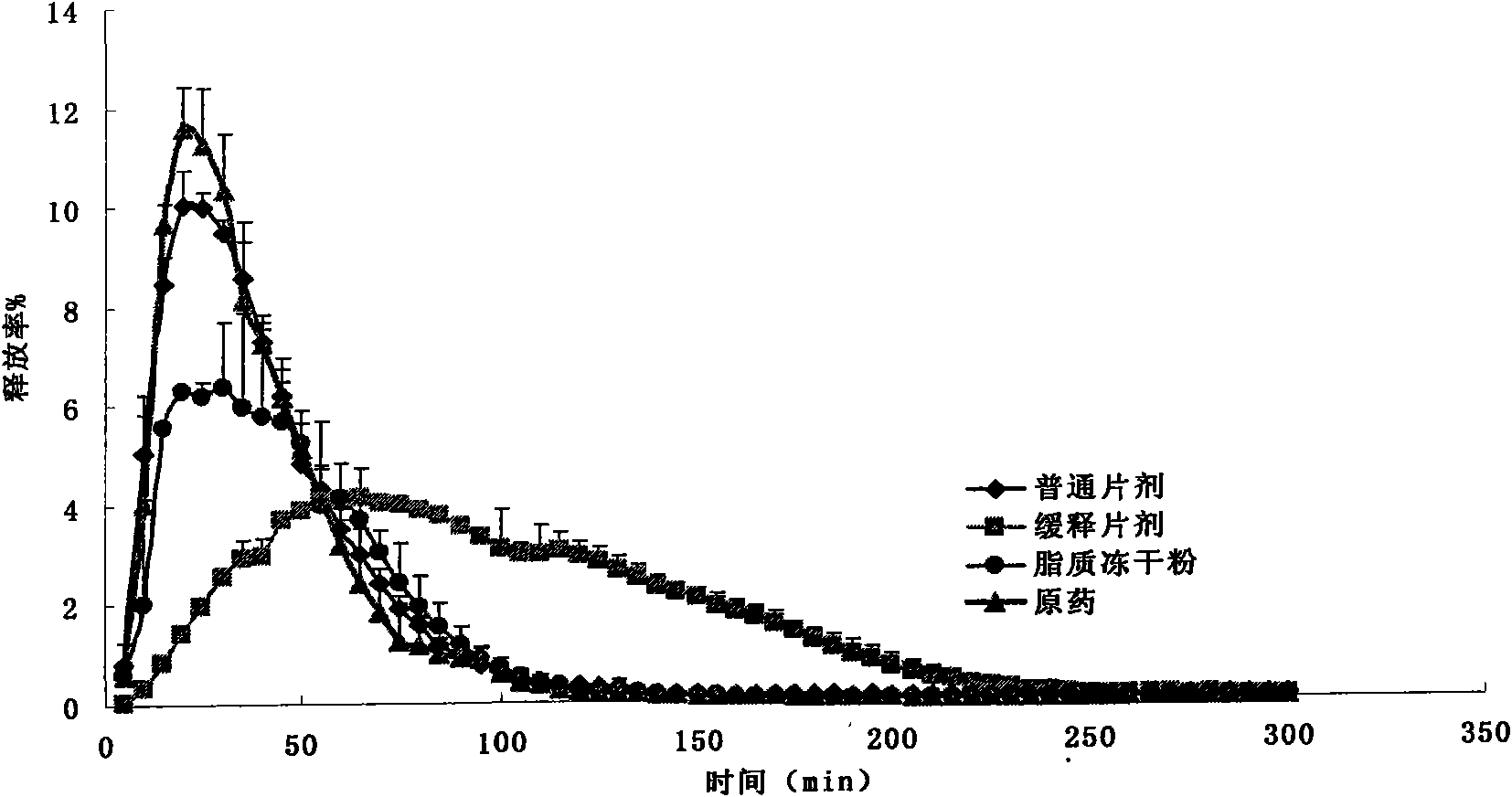
Embodiment 1
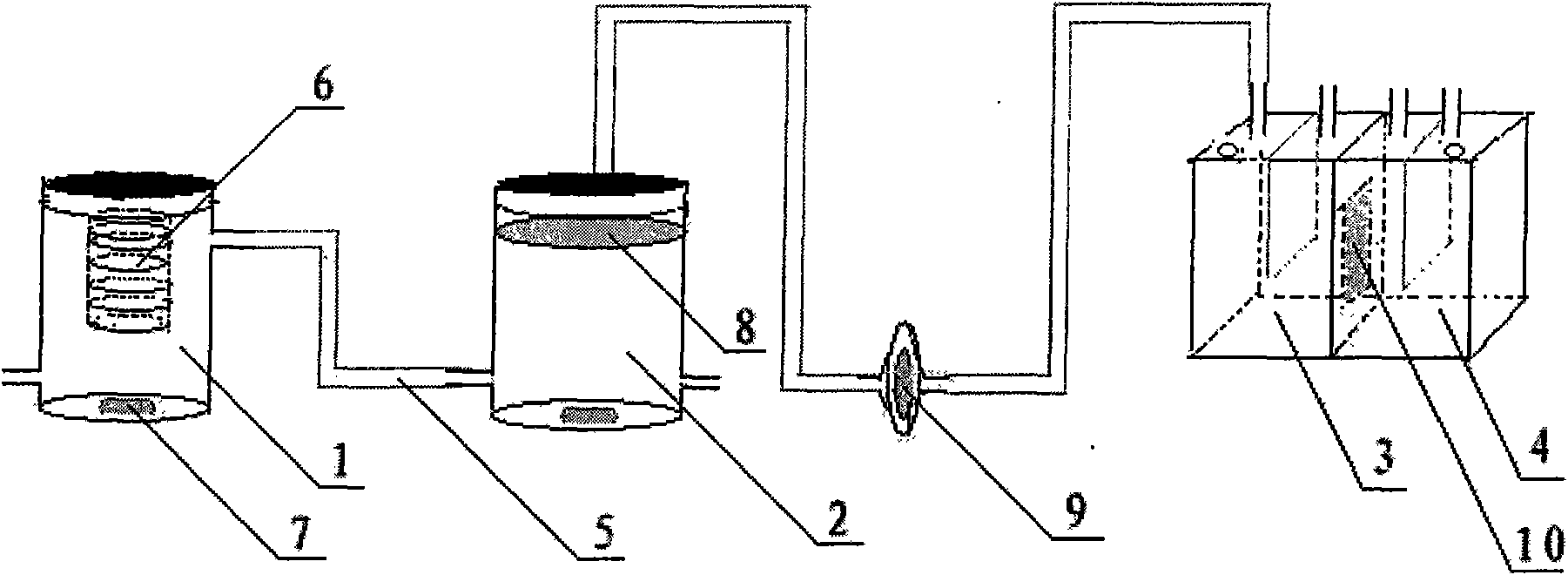
[0038] A device for evaluating the dissolution / absorption process of solid drug preparations, mainly composed of a drug dissolution chamber 1, a pH adjustment chamber 2 and a diffusion pool, wherein the bottom of the drug dissolution chamber 1 is provided with a magnetic stirrer 7, and the diffusion pool is composed of a supply chamber 3 , a receiving chamber 4 and a rat isolated intestinal tube 10' fitted between the two, each chamber is sequentially connected by a silicone tube 5 with an inner diameter of 1.0mm, and a drug-loading basket 6 is also provided in the drug dissolution chamber. There is a distance between the drug-loaded basket 6 and the magnetic stirrer 7 at the bottom of the drug dissolution chamber 1, the upper end of the drug-loaded basket 6 is fixedly connected to the upper end of the drug dissolution chamber 1, and the central axis of the drug-loaded basket 6 is connected to the drug dissolution chamber 1. The positions of the central axes coincide, and the d...
Embodiment 2
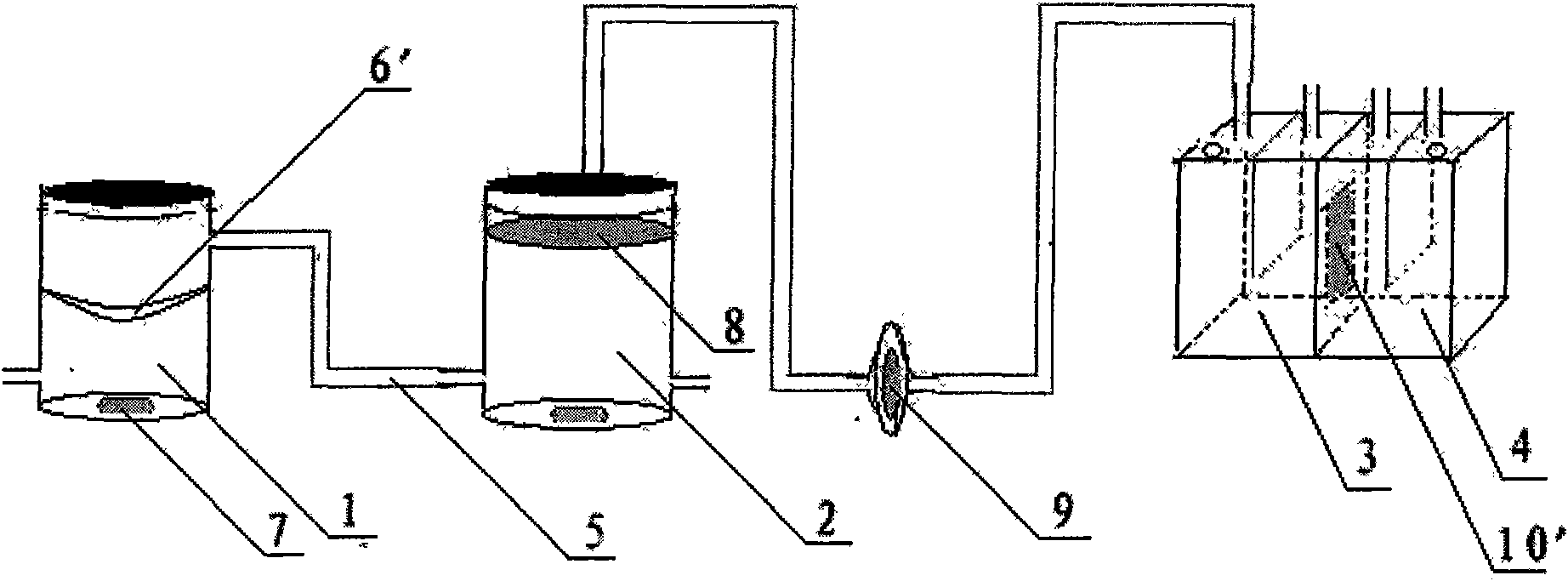
[0040] A device for evaluating the dissolution / absorption process of solid drug preparations, mainly composed of a drug dissolution chamber 1, a pH adjustment chamber 2 and a diffusion pool, wherein the bottom of the drug dissolution chamber 1 is provided with a magnetic stirrer 7, and the diffusion pool is composed of a supply chamber 3 , the receiving chamber 4 and the Caco-2 monolayer cell membrane 10 embedded between the two, each chamber is connected in turn by a silicone tube 5 with an inner diameter of 2.0 mm, and a metal clip is also provided in the drug dissolving chamber 1 6', the two ends of the metal sheet clip 6' are fixed on the side wall of the drug dissolution chamber 1, and its center coincides with the midpoint of the central axis of the drug dissolution chamber 1. The metal sheet clip 6' is made of strong acid-resistant, strong-resistant Alkali, high temperature resistant stainless steel material.
Embodiment 3
[0042] A device for evaluating the dissolution / absorption process of solid drug preparations, mainly composed of a drug dissolution chamber 1, a pH adjustment chamber 2 and a diffusion pool, wherein the bottom of the drug dissolution chamber 1 is provided with a magnetic stirrer 7, and the diffusion pool is composed of a supply chamber 3 , the receiving chamber 4 and the Caco-2 monolayer cell membrane 10 embedded between the two, each chamber is connected in turn by a silicone tube 5 with an inner diameter of 1.5 mm, and a drug-loading basket is also provided in the drug dissolution chamber 1 6. There is a distance between the drug-loaded basket 6 and the magnetic stirrer 7 at the bottom of the drug dissolution chamber 1, the upper end of the drug-loaded basket 6 is fixedly connected to the upper end of the drug dissolution chamber 1, and the central axis of the drug-loaded basket 6 is in line with the drug dissolution chamber. The central axis of chamber 1 coincides with each ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com