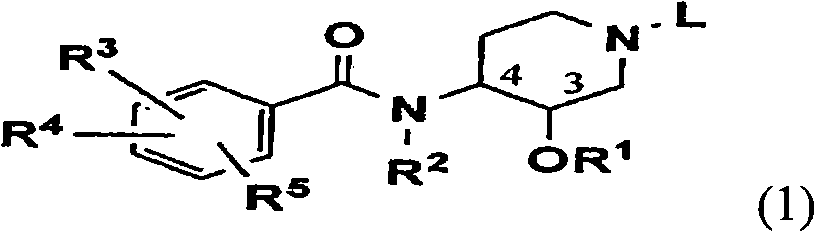
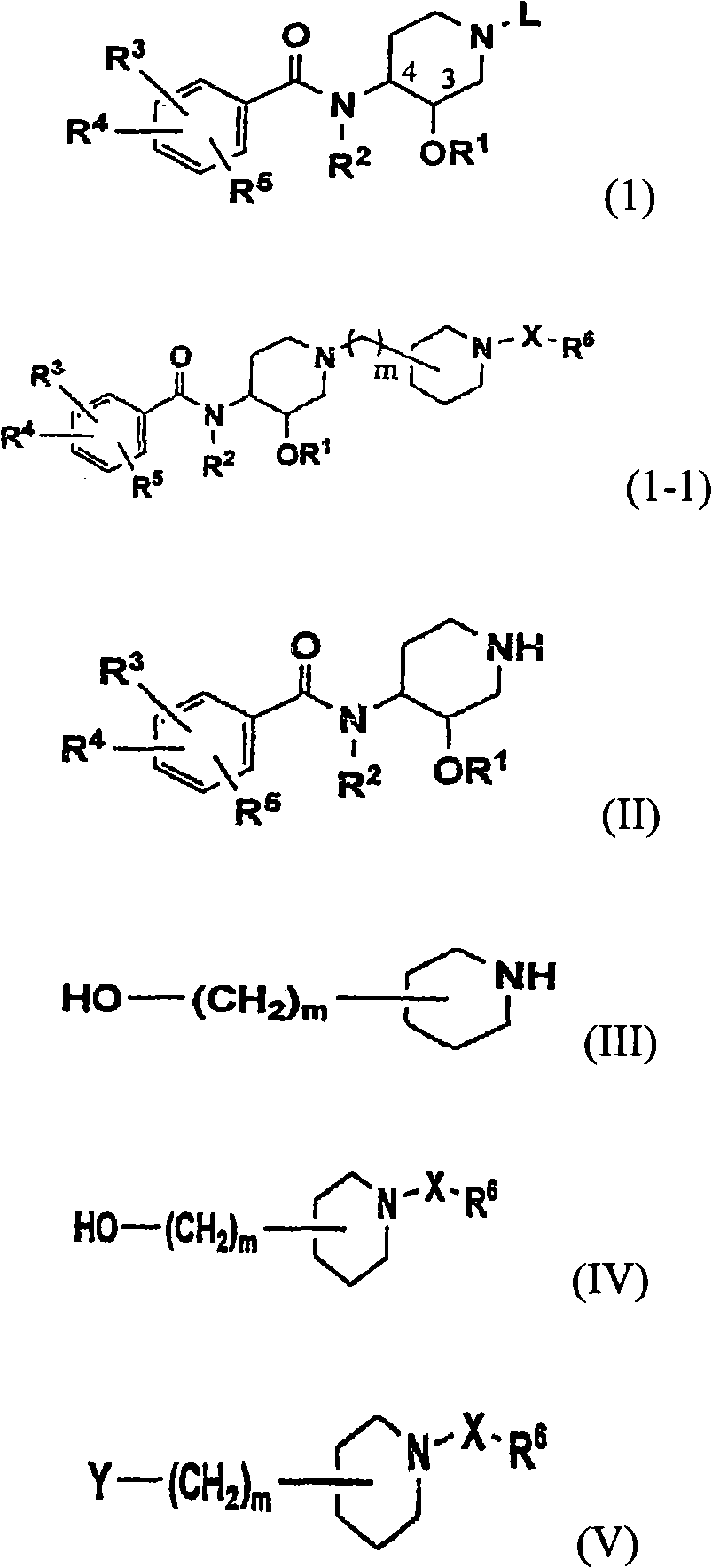
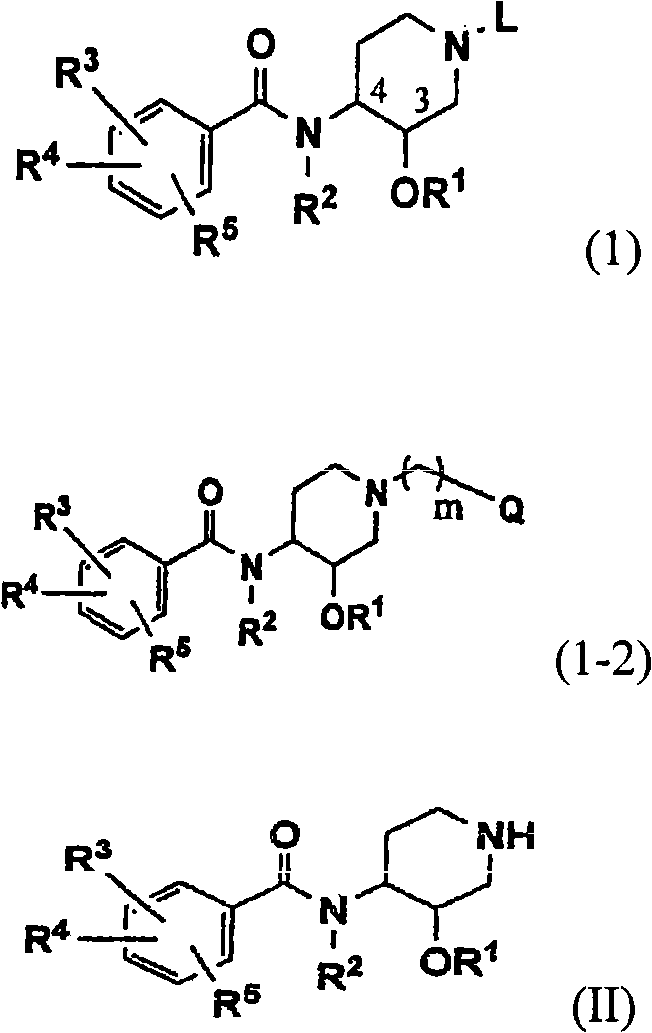
Novel benzamide derivatives and process for the preparation thereof
A kind of technology of methoxybenzamide and methoxybenzamide group, applied in the field of benzamide derivatives and preparation thereof, and can solve problems such as safety restrictions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0156] Example 1: Preparation of 4-[(cis-4-(4-amino-5-chloro-2-methoxybenzamido)-3-methoxypiperidin-1-yl)methyl Ethyl]piperidine-1-carboxylate
[0157] Step 1: Preparation of ethyl 4-(hydroxymethyl)piperidine-1-carboxylate
[0158] 15 g of 4-piperidinemethanol were dissolved in dichloromethane, and the solution was cooled to 0°C. Next, add 38.4 mL of triethylamine (Et 3 N) followed by the slow addition of 13.7 mL of ethyl chloroformate. The reaction mixture was warmed to room temperature, stirred for 3 hours, then extracted with dichloromethane. The extracted organic layer was dissolved in anhydrous magnesium sulfate (MgSO 4 ), concentrated under reduced pressure, and purified by column chromatography to afford 12 g (49%) of the title compound.
[0159] 1 H NMR (CDCl 3 ): δ4.23-4.08(m, 4H), 3.49(d, J=6.0Hz, 2H), 2.80-2.68(m, 2H), 1.76-1.60(m, 3H), 1.24(t, J=7.2Hz , 3H), 1.20-1.08(m, 2H)
[0160] Step 2: Preparation of ethyl 4-(bromomethyl)piperidine-1-carboxylat...
Embodiment 2
[0167] Example 2: Preparation of 4-[((3S, 4R)-4-(4-amino-5-chloro-2-methoxybenzamido)-3-methoxypiperidin-1-yl)methanol Ethyl]piperidine-1-carboxylate
[0168] Similar to Example 1, from 485 mg of 4-piperidinemethanol, 0.4 mL of ethyl chloroformate and 400 mg of 4-amino-5-chloro-2-methoxy-N-((3S, 4R)-3- Methoxypiperidin-4-yl)benzamide yielded 208 mg of the title compound (hereinafter referred to as "(+)-norcisapride").
[0169] [α] 25 D =+11.5 (c=0.5, MeOH)
Embodiment 3
[0170] Example 3: Preparation of 4-[2-(cis-4-(4-amino-5-chloro-2-methoxybenzamido)-3-methoxypiperidin-1-yl)ethyl Ethyl]piperidine-1-carboxylate
[0171] Similar to Example 1, 157 mg of the title compound were prepared from 591 mg of 4-piperidineethanol, 0.73 mL of ethyl chloroformate, and 300 mg of cis-norcisapride.
[0172] 1 H NMR (CDCl 3 ): δ8.16(d, J=8.4Hz, 1H), 8.01(s, 1H), 6.25(s, 1H), 4.49(bs, 2H), 4.15-3.96(m, 4H), 3.80(s, 3H), 3.37(bs, 4H), 3.04-2.96(m, 1H), 2.75-2.61(m, 3H), 2.41-2.24(m, 3H), 2.16-2.00(m, 2H), 1.85-1.71( m, 2H), 1.65-1.56(m, 2H), 1.48-1.33(m, 3H), 1.19(t, J=6.8Hz, 3H), 1.15-1.00(m, 2H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com