Preparation method of core-shell Fe/Pd bimetallic nano-catalyst
A bimetallic, core-shell type technology, applied in the direction of catalyst activation/preparation, metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, etc. High production cost and other issues, to achieve good dechlorination effect, high electron transport performance, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation method of core-shell type nano bimetallic Fe / Pd catalyst:
[0029] First polish the glassy carbon electrode with 3500# metallographic sandpaper, and then polish the mirror surface of the paper with 0.5μm alumina powder. Before the experiment started, the glassy carbon electrode was first placed in HNO 3 Solution (HNO 3 :H 2 (O volume ratio 1:1) ultrasonic 5min, and then rinse with ultrapure water. The electrochemical experiments were all carried out in the electrolytic cell of the traditional three-electrode system: a clean glassy carbon electrode was used as the working electrode, a Pt sheet was used as the counter electrode, and a saturated calomel electrode was used as the reference electrode.
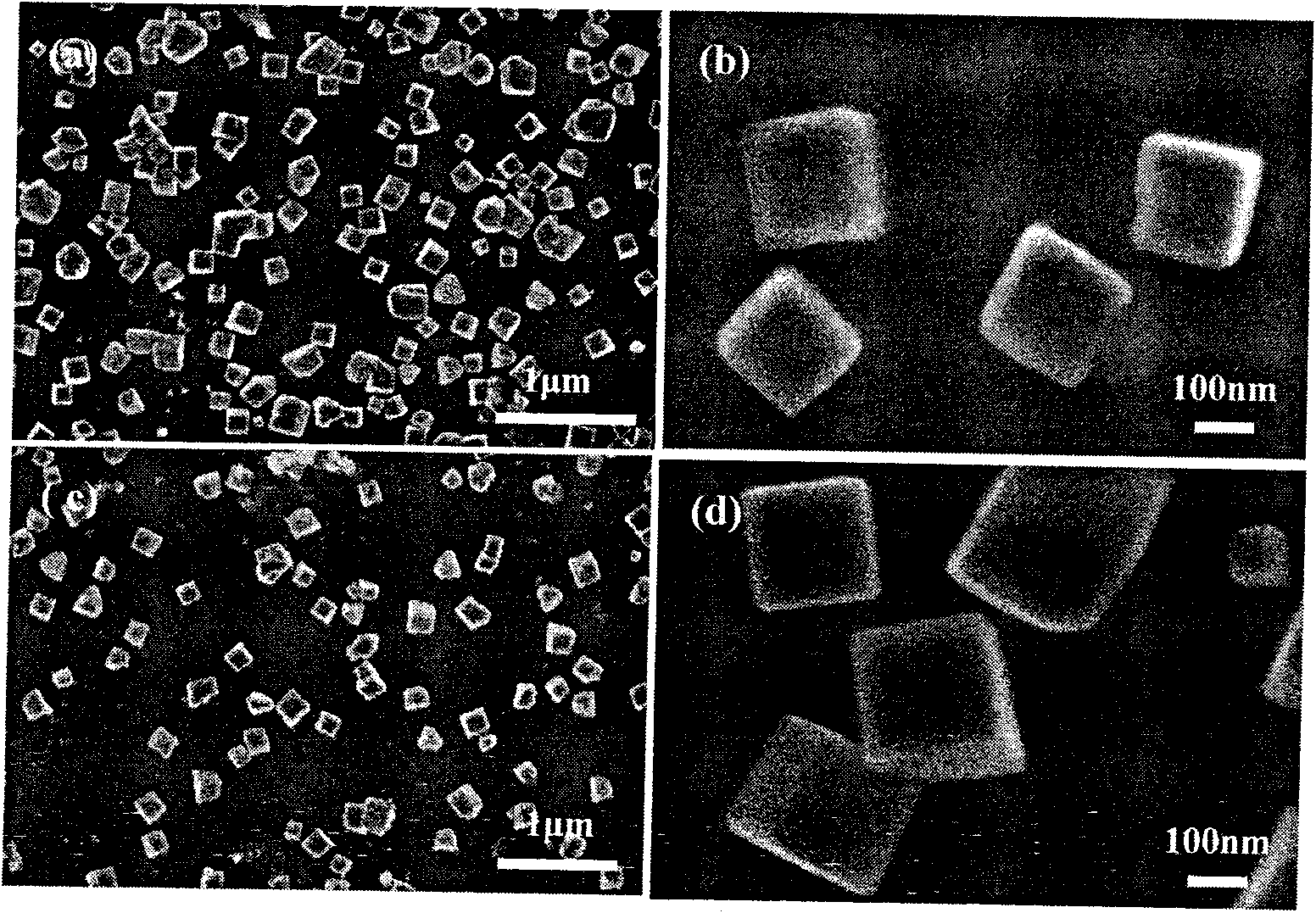
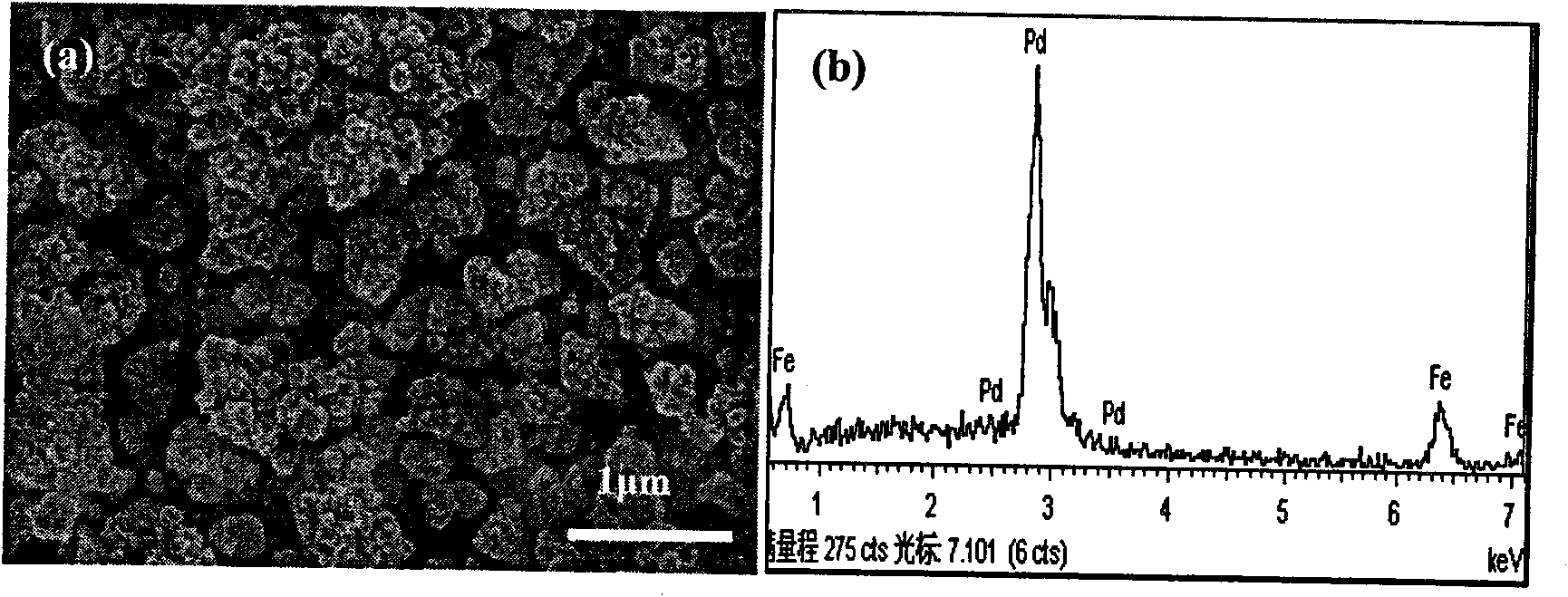
[0030] NaCl and FeCl 2 The mixed solution of NaCl is used as the deposition solution, and the concentration of NaCl is 0.25mol dm -3 , FeCl 2 The concentration is 10mmol dm -3 , control the temperature at 30°C, and deposit a large amount of Fe on the gl...
Embodiment 2
[0033] The preparation method of core-shell type nano bimetallic Fe / Pd catalyst:
[0034] First polish the glassy carbon electrode with 3500# metallographic sandpaper, and then polish the mirror surface of the paper with 0.5μm alumina powder. Before the experiment started, the glassy carbon electrode was first placed in HNO 3 (HNO 3 :H 2 (O volume ratio 1:1) ultrasonic 5min, and then rinse with ultrapure water. The electrochemical experiments were all carried out in the electrolytic cell of the traditional three-electrode system: a clean glassy carbon electrode was used as the working electrode, a Pt sheet was used as the counter electrode, and a saturated calomel electrode was used as the reference electrode.
[0035] NaCl and FeCl 2 The mixed solution of NaCl is used as the deposition solution, and the concentration of NaCl is 0.25mol dm -3 , FeCl 2 The concentration is 10mmol dm -3 , control the temperature at 30°C, and deposit a large amount of Fe on the glassy carb...
Embodiment 3
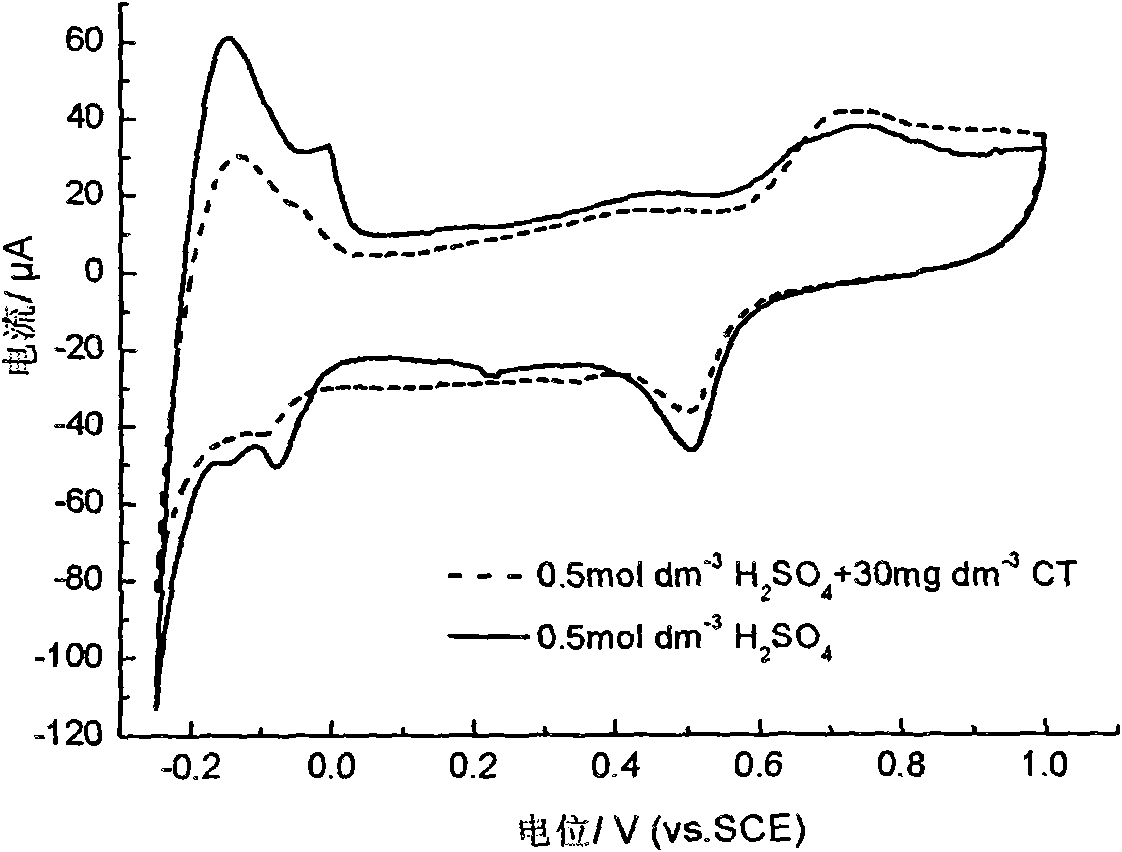
[0038] The core-shell type nano-bimetallic Fe / Pd catalyst that embodiment 1 makes is respectively in 0.5mol dm -3 h 2 SO 4 and 0.5moldm -3 h 2 SO 4 +30mg dm -3 CCl 4 A voltammetric scan was performed, with the addition of CCl by contrast 4 The cyclic voltammetry curves before and after in sulfuric acid solution found that adding CCl 4 After, due to CCl 4 The adsorption of molecules on the surface of the electrode occupies the active sites of hydrogen adsorption, so that the peaks of hydrogen adsorption and oxidation at positive potentials disappear, and the peaks of hydrogen formation and desorption at negative potentials decrease, such as image 3 shown.
[0039] According to the different forms of hydrogen on Pd at different potentials, that is, the difference in hydrogen activity at different potentials, at 0.5moldm -3 h 2 SO 4 with 30mg dm -3 CCl 4 In the mixed solution, by changing the constant potential electrolysis potential (dechlorination time is 30min, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com