Novel prophylactic and/or therapeutic agent for diabetic neuropathy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
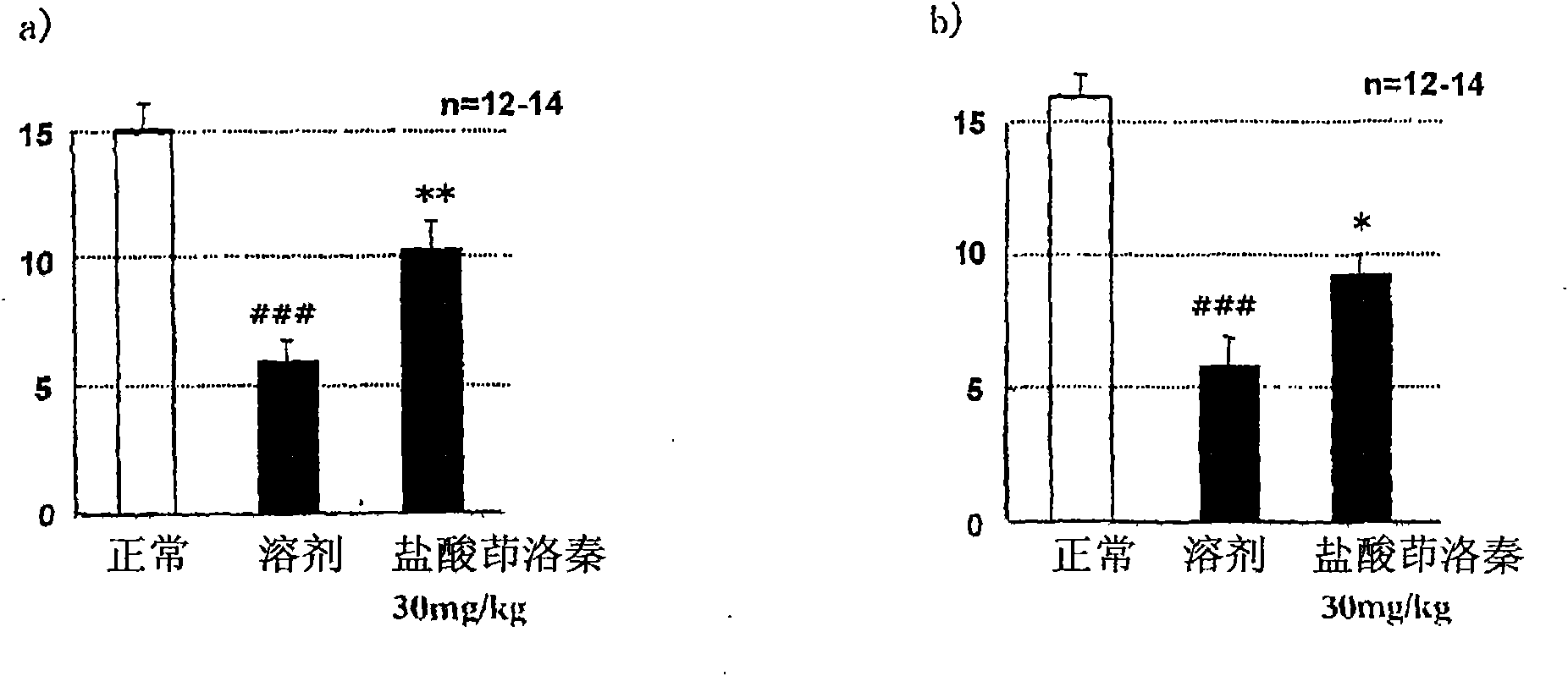
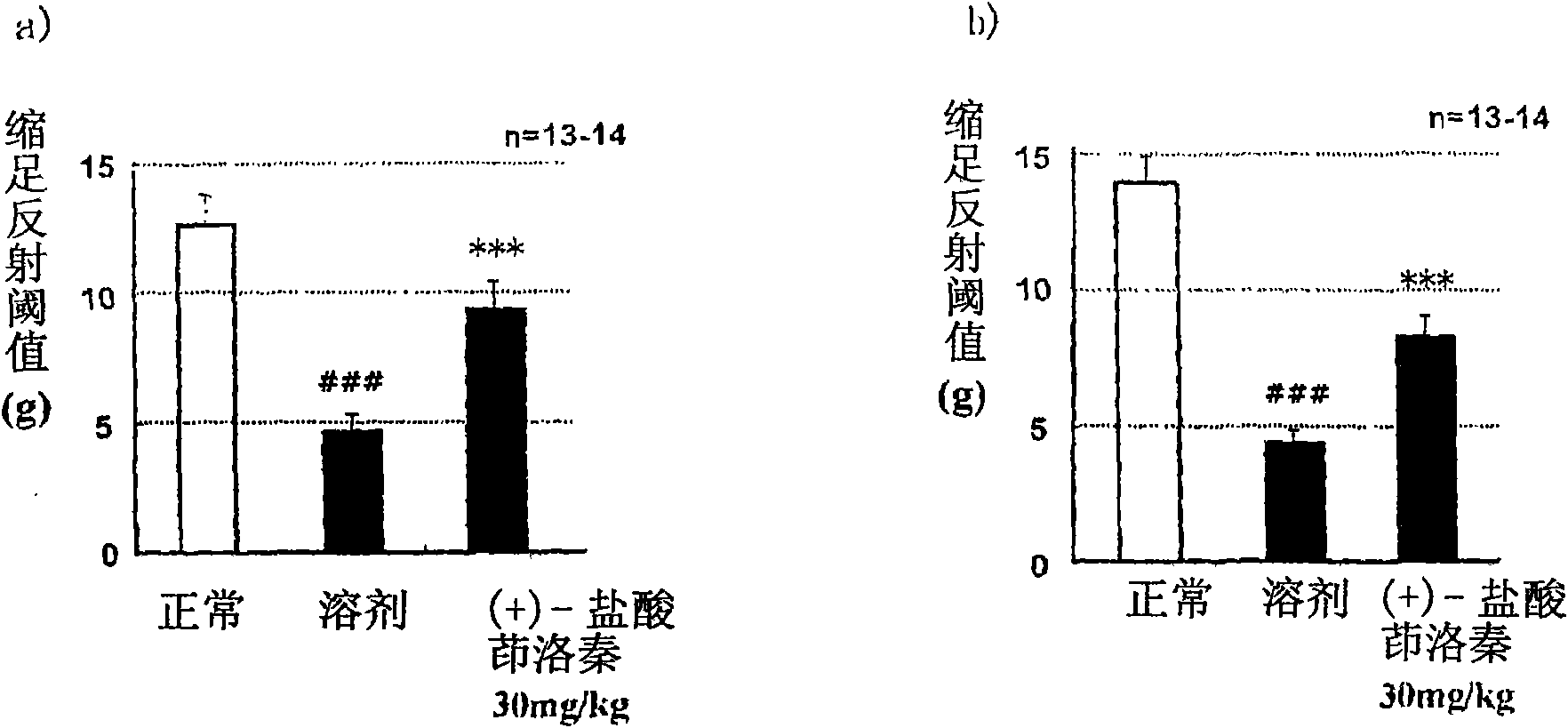
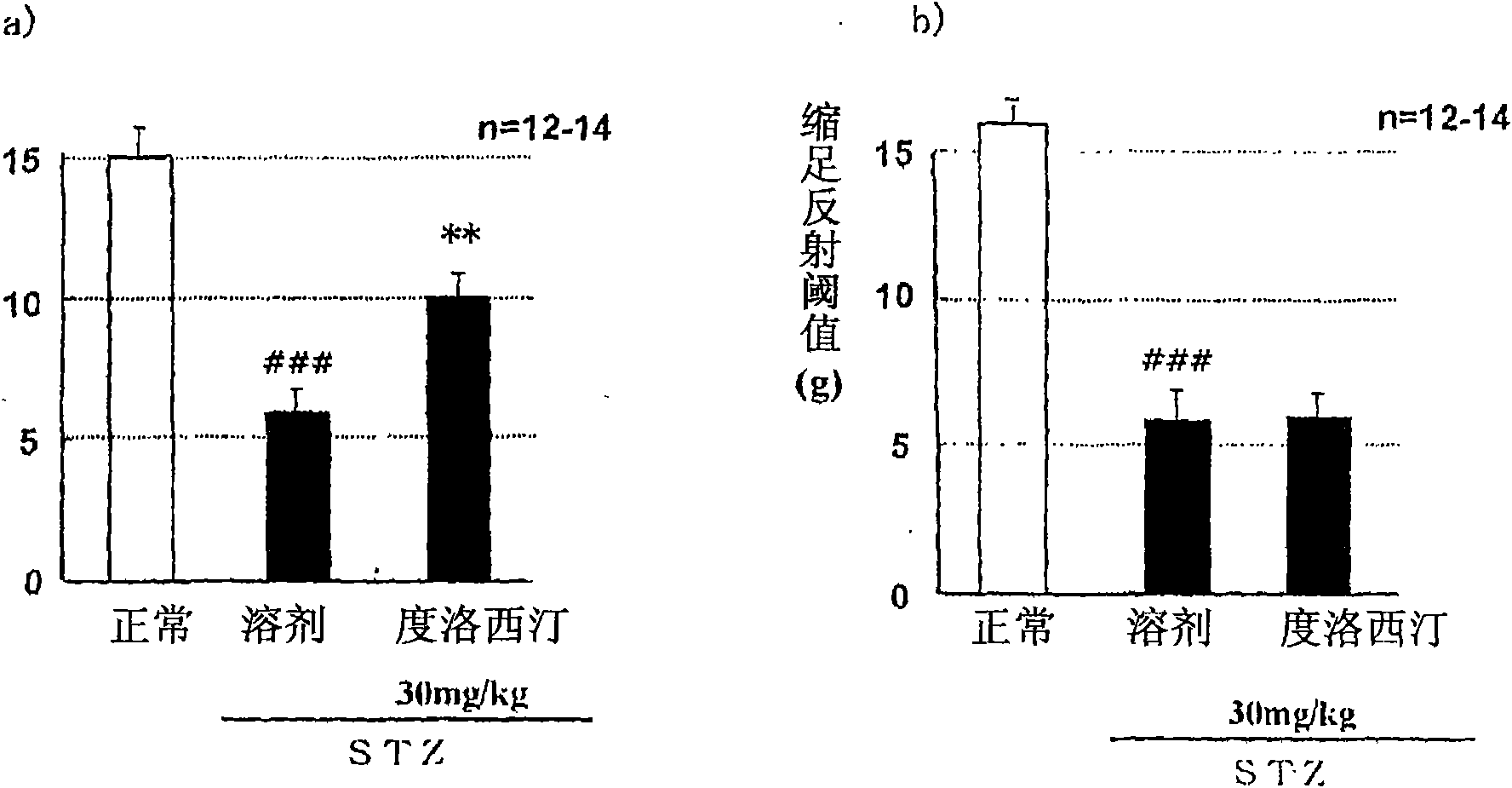
[0088] STZ (streptozotocin) induced diabetic rats were prepared by the following steps. At 7 weeks of age, STZ (45 mg / kg) was administered intravenously. In the second week after STZ administration, blood was collected from the tail vein and the blood glucose level was measured, and it was found that the blood glucose level had increased by more than 300 mg / dL. According to the weight, blood sugar level, and the average value of the pain response threshold measured the day before the administration, the administration groups are divided into groups so that the deviation of each group is small. Separately prepare the non-administered STZ group as the normal group. The test drug was administered orally once a day for 28 consecutive days. According to the Von Frey Test (Pain Threshold Test), sensory abnormalities caused by neurological disorders were measured at two time points, one hour after the end of the administration on the 28th day and the 7th day after the end of the adm...
Embodiment 2
[0092] In order to verify whether (+)-2-[(inden-7-yloxy)methyl]morpholine·hydrochloride can cure the neurological disorder itself, we studied (+)-2-[ in STZ-induced diabetic rats (Indene-7-yloxy)methyl]morpholine hydrochloride improves motor nerve conduction velocity (MNCV). The method of Cameron et al. (The Journal of Experimental Physisology 1989, Vol. 74, p917-926) was partially modified to perform this measurement.
[0093] The animals used in the measurement were the rats in the solvent administration group in Example 2 and the 30 mg / kg administration group on the 7th or 8th day after the continuous administration was terminated, respectively. In addition, the same test was performed on the normal group. Rats were anesthetized with phenobarbital sodium, and the rectal temperature was maintained at 37.5°C in a body temperature maintaining device for small animals, and MNCV was measured using an evoked potential measuring device. First, install stimulating positive electrode...
Embodiment 3
[0105] In order to verify whether (+)-2-[(inden-7-yloxy)methyl]morpholine·hydrochloride acts on the expression of neurotrophic factors, we studied (+)-2-[(inden-7- (Oxyoxy)methyl]morpholine·hydrochloride improves the expression of neurotrophic factors in the spinal cord and heel nerve nodes of STZ-induced diabetic rats.
[0106] The neuronutrition of the animals on the 7th or 8th day after the end of continuous administration was measured in the normal group, the vehicle administration group of STZ-induced diabetic rats, and the 30mg / kg administration group in Example 2 respectively. Factor expression. The lumbar spinal cord and heel ganglia (L4, L5, L6) were extracted, and total RNA was extracted using RNA extraction kit RNeasy (Qiagen). The extracted RNA is used as a template for in vitro reverse transcription reaction to obtain cDNA. Adopted for fibroblast growth factor 2 (FGF-2), insulin-like growth factor 1 (IGF-1) and glyceraldehyde 3-phosphate dehydrogenase (Glyceraldehy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com