Copper and silicon catalyst for preparing ethanediol by hydrogenating dimethyl oxalate and preparation method thereof
A technology of dimethyl oxalate and catalyst is applied in the field of copper-silicon catalyst and preparation, and achieves the effects of being beneficial to industrial application, easy to operate, and high ethylene glycol selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: Tetraethyl orthosilicate is the Cu that silicon source ethanol is co-solvent 0.3 Si-TEOS-E catalyst
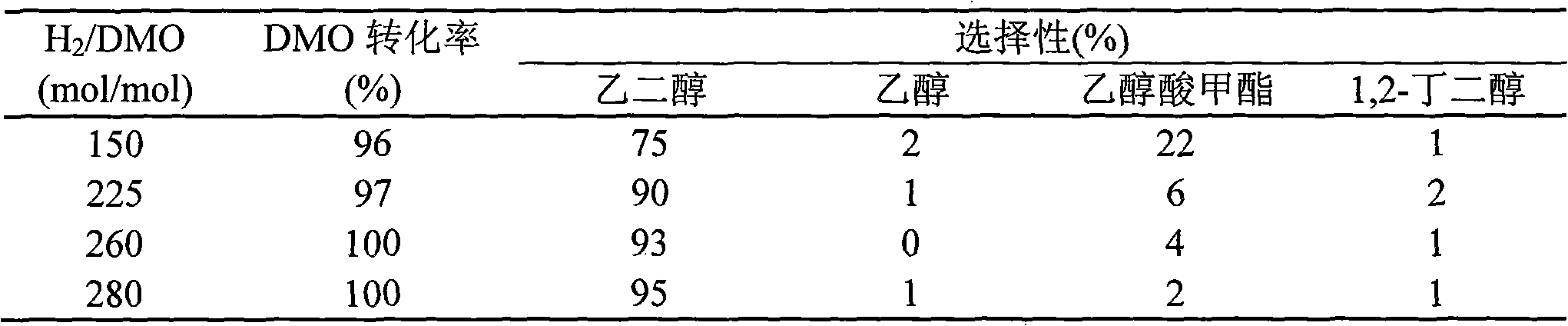
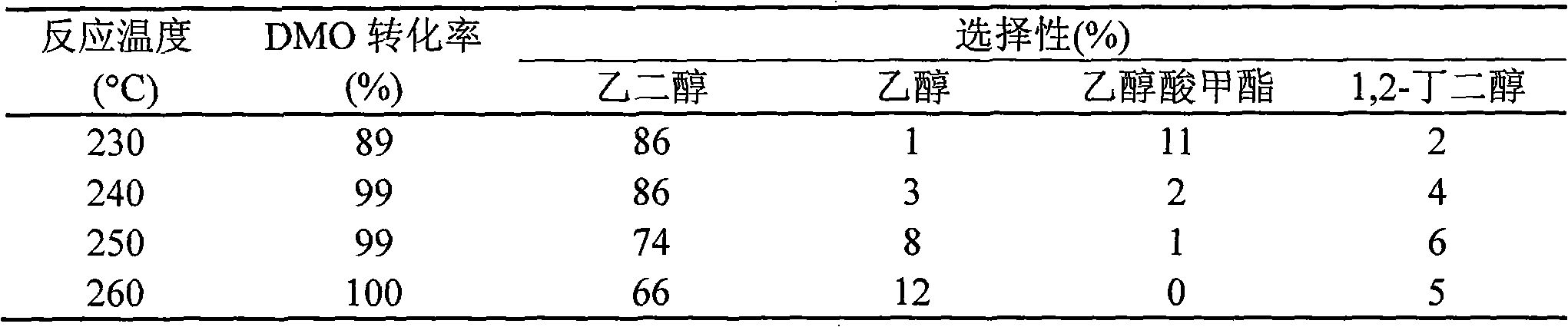
[0020] Under stirring, 8.5g Cu(NO 3 ) 2 ·3H 2 O was dissolved in 75 mL of water, and 25 mL of ethanol was added as a co-solvent. Then add ammonia water dropwise to the above solution until the pH value reaches 12-13, and continue stirring for 0.5 h after the dropwise addition to obtain a water-alcohol mixed solution of the copper ammonia complex. Then, 24.43 g of ethyl orthosilicate was added dropwise to the above mixed solution, and after stirring for 6 hours, it was aged at room temperature for 12 hours. The resulting mixture was heated and evaporated to remove most of the water, alcohol and ammonia, and then filtered, and the obtained solid was washed 5 times with water, dried at 120°C for 15 hours, and calcined at 450°C for 4 hours to obtain the catalyst precursor. The catalyst precursor was treated with 50 mL / min of H in a fixed-bed reactor 2 Af...
Embodiment 2
[0021] Embodiment 2: Ethyl orthosilicate is Cu that silicon source ethanol is co-solvent 0.05 Si-TEOS-E catalyst
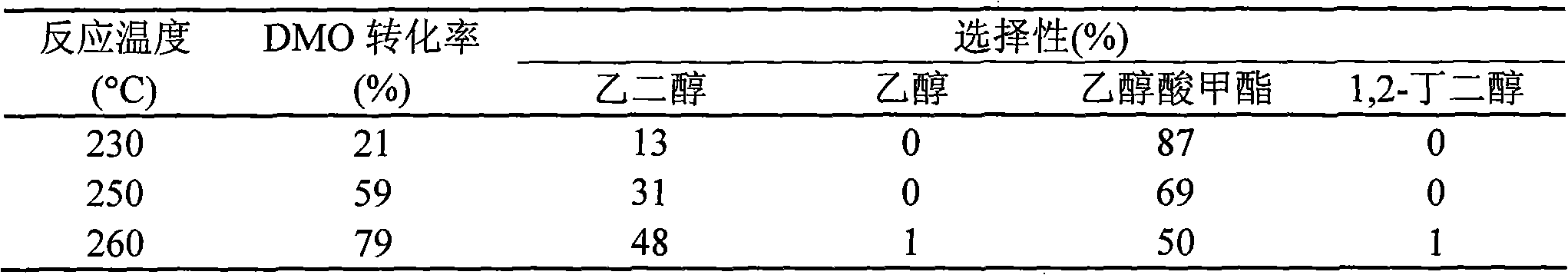
[0022] Under stirring, 4.5g Cu(NO 3 ) 2 ·3H 2 O was dissolved in 25 mL of water, and 125 mL of ethanol was added as a co-solvent. Then add ammonia water dropwise to the above solution until the pH value reaches 12-13, and continue stirring for 0.5 h after the dropwise addition to obtain a water-alcohol mixed solution of the copper ammonia complex. Then, 77.61 g of ethyl orthosilicate was added dropwise to the above mixed solution, and after stirring for several 6 hours, it was aged at room temperature for 6 hours. Other preparation conditions are the same as in Example 1, and the test results of the hydrogenation reaction activity of dimethyl oxalate are shown in Table 2.
Embodiment 3
[0023] Embodiment 3: Ethyl orthosilicate is silicon source methanol is the Cu of co-solvent 0.2 Si-TEOS-M catalyst
[0024] Under stirring, 8.5g Cu(NO 3 ) 2 ·3H 2 O was dissolved in 150 mL of water, and 25 mL of methanol was added as a co-solvent. Then add ammonia water dropwise to the above solution until the pH value reaches 12-13, and continue stirring for 0.5 h after the dropwise addition to obtain a water-alcohol mixed solution of the copper ammonia complex. Add 36.65 g of ethyl orthosilicate dropwise to the above mixed solution, continue stirring for 6 hours, and then age at room temperature for 16 hours. Other preparation conditions are the same as in Example 1, and the test results of the hydrogenation reaction activity of dimethyl oxalate are shown in Table 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com