Plating layer for electrochemistry corrosion resistant electronic encapsulation shell
An electronic packaging and electrochemical technology, applied in the direction of circuits, coatings, electrical components, etc., can solve the problems of glass easy to fall off, serious corrosion of the shell, breakage, etc., and achieve the effect of meeting the requirements of oxidizability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
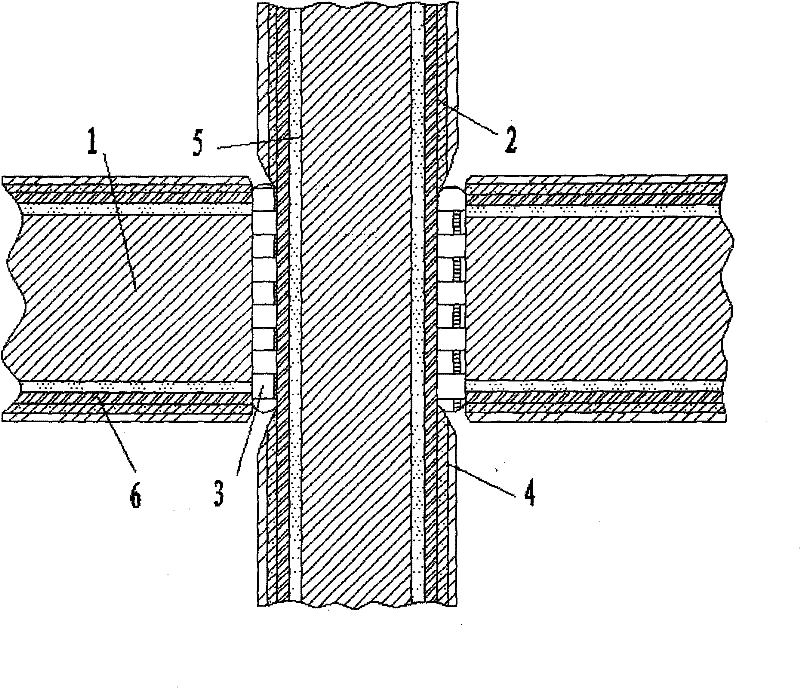
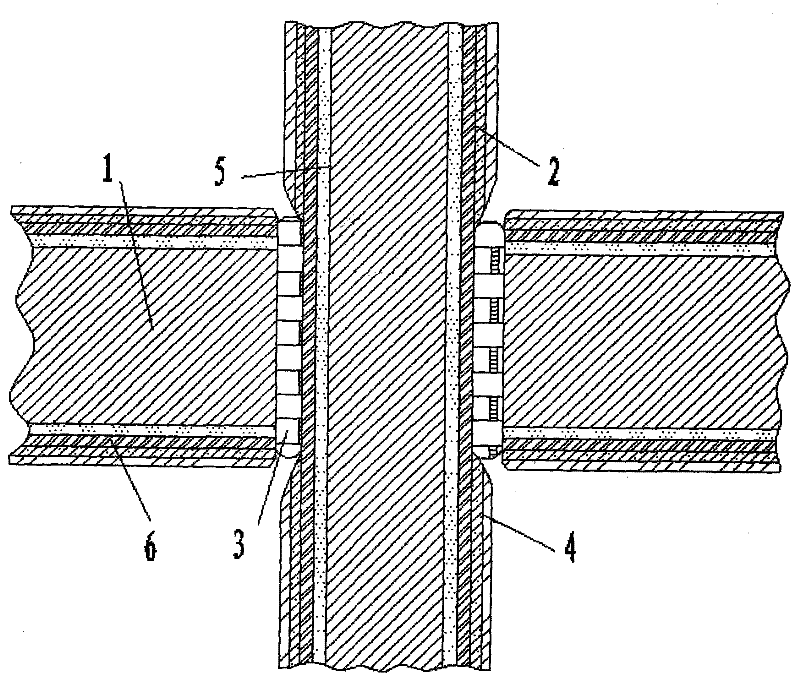
[0014] See attached picture.
[0015] It is used for the coating of the electronic package housing to resist electrochemical corrosion. The surface layer of the housing base 1 and the surface layer of the lead wire 7 are sequentially plated with an inert metal layer 5, an iron-nickel layer 6, a nickel layer 2 and a gold layer 4 from the inside to the outside. The weight percent of iron in the iron-nickel layer 6 is 15%. Both the lead wire 7 and the housing base 1 are pre-plated with an inert metal layer 5 before sintering the frit glass layer 3. The inert metal can be selected from gold, silver, copper, etc. to form a high potential barrier layer at the bottom. The iron-nickel layer 6 is pre-plated again, and the iron-nickel layer 6 can play a sacrificial corrosion effect in salt spray corrosion, thereby effectively protecting the body material; the weight percentage of the iron-nickel layer 6 iron is 15%, and the coating and Kovar The composition is basically the same, which...
Embodiment 2
[0017] It is used for the coating of the electronic package housing to resist electrochemical corrosion. The surface layer of the housing substrate 1 and the surface layer of the lead wire 7 are sequentially plated with an iron-nickel layer 6, a nickel-plated layer 2 and a gold-plated layer 4 from the inside to the outside, and the iron-nickel layer 6 is iron. The weight percentage is 15%. The iron-nickel alloy layer 6 can play a sacrificial corrosion role in salt spray corrosion, thereby effectively protecting the body material; the weight percentage of the iron-nickel layer 6 iron is 15%, and the composition of the coating is basically the same as that of Kovar alloy, which meets the matching requirements. The basic requirements of permanent sealing and meet the requirements of substrate surface oxidability.
Embodiment 3
[0019] It is used for the coating of the electronic package housing to resist electrochemical corrosion. The surface layer of the housing base 1 and the surface layer of the lead wire 7 are sequentially plated with an inert metal layer 5, an iron-nickel layer 6, a nickel layer 2 and a gold layer 4 from the inside to the outside. The weight percent of iron in the iron-nickel layer 6 is 65%. All the other are with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com