Amphiphilic hyper-branched thioxanthone photoinitiator and preparation method thereof
An amphiphilic hyperbranched, xanthone light technology, applied in the field of photochemistry, can solve the problems of odor, toxicity, migration, and volatility, and achieve the effects of reducing toxicity, improving photo-initiating performance, and improving solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1. Slowly add 1.54g (0.01mol) of thiosalicylic acid into 15ml of concentrated sulfuric acid at room temperature, slowly add 5.0g (0.055mol) of phenol under the condition of full stirring, and the addition time is 30 minutes, then Stir at room temperature for 1 hour, raise the temperature to 80° C. for 2 hours, then cool down to room temperature and let stand for 12 hours. The reaction mixture was slowly poured into 150ml of boiling water, then further boiled for 10 minutes, filtered after cooling, washed three times with water, and then reconstituted in a mixed solvent with a volume ratio of 1,4-dioxane:water=4:1 Crystallization gives 2-hydroxythioxanthone. Add 2.3g (0.01mol) of 2-hydroxythioxanthone, 15ml of epichlorohydrin, 5g (0.036mol) of anhydrous potassium carbonate, 15ml of toluene and 0.5ml of polyethylene glycol (molecular weight 400) into a three-necked flask , stirred at 80°C for 2 hours, then refluxed in an oil bath at 135°C for 1 hour, poured out the organ...
Embodiment 2
[0035] Steps 1 and 2 are the same as in Example 1.
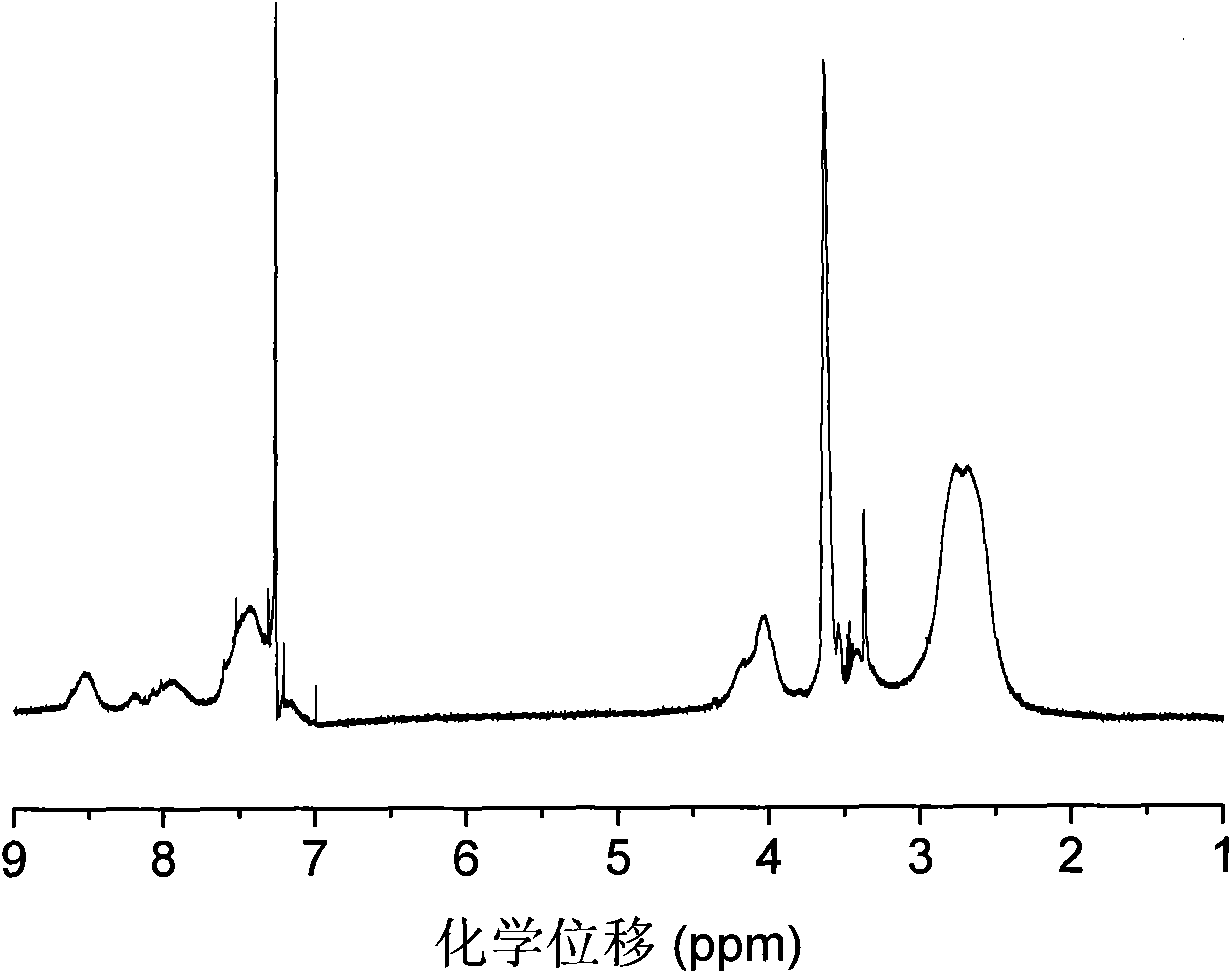
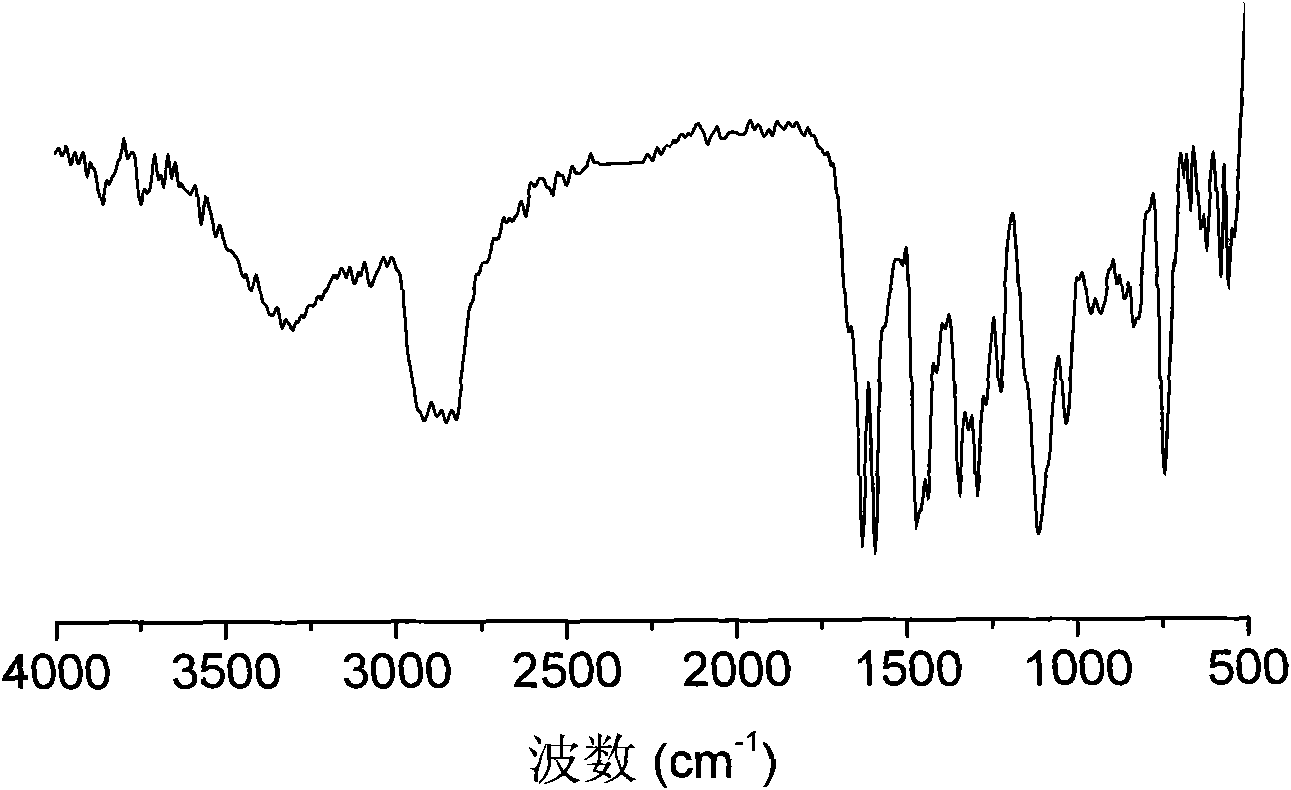
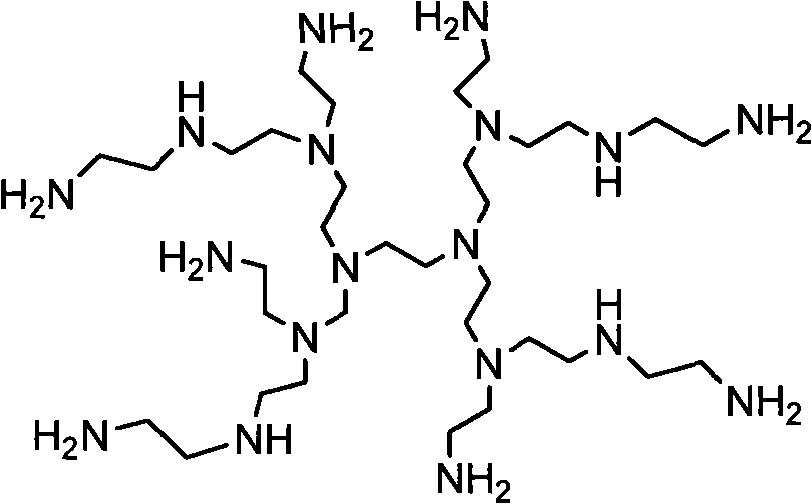
[0036] 3. Mix 0.284g (1mmol) 2ETX, 0.108g (0.2mmol) polyethyleneimine (M n =600) and 10ml ethanol were added in the there-necked flask, after dissolving completely, the solution was reacted at 80°C for 24 hours under nitrogen protection, then added 0.081g (0.2mmol) polyethylene glycol monomethyl ether containing epoxy groups, Continue to react at 80°C for 24 hours under the protection of nitrogen, then precipitate into anhydrous ether 10 times the volume of the reaction solution, pour out the solvent, and dry in vacuum to obtain the target product amphiphilic hyperbranched thioxanthone photoinitiator . 1 H NMR (CDCl 3 ): δ=8.58-7.21 (aromatic), 4.10-3.80 (-OCH 2 , -OCH), 3.72-3.40 (-OCH 2 CH2 ), 3.35 (-OCH 3 ), 2.8-2.4 (-NCH 2 CH 2 ).FTIR(KBr): 3326(-OH), 2880(-CH), 1630(C=O).
Embodiment 3
[0038] 1. Slowly add 1.54g (0.01mol) of thiosalicylic acid into 15ml of concentrated sulfuric acid at room temperature, slowly add 5.4g (0.05mol) of m-cresol under the condition of sufficient stirring, and the addition time is 30 minutes , then stirred at room temperature for 1 hour, heated to 80° C. for 2 hours, and then stood overnight at room temperature. The reaction mixture was slowly poured into 150ml of boiling water, then further boiled for 10 minutes, filtered after cooling, washed three times with water, and then reconstituted in a mixed solvent with a volume ratio of 1,4-dioxane:water=4:1 Crystallization affords 4-methyl-2-hydroxythioxanthone. Then 2.3g (0.01mol) of 4-methyl-2-hydroxythioxanthone, 15ml epichlorohydrin, 5g (0.036mol) of anhydrous potassium carbonate, 15ml toluene and 0.5ml polyethylene glycol (molecular weight 400) into a three-necked flask, stirred at 80°C for 2 hours, then refluxed in an oil bath at 135°C for 1 hour, poured out the organic layer a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com