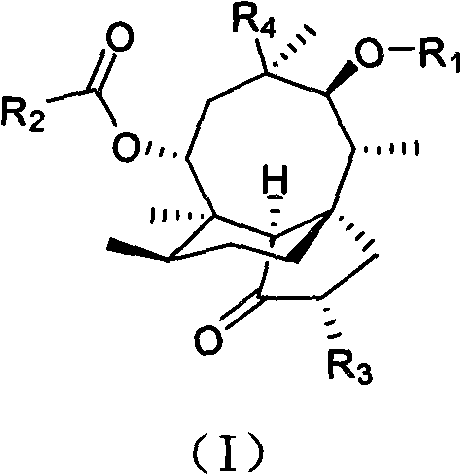
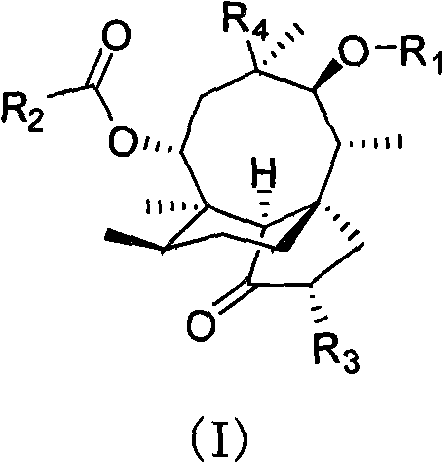
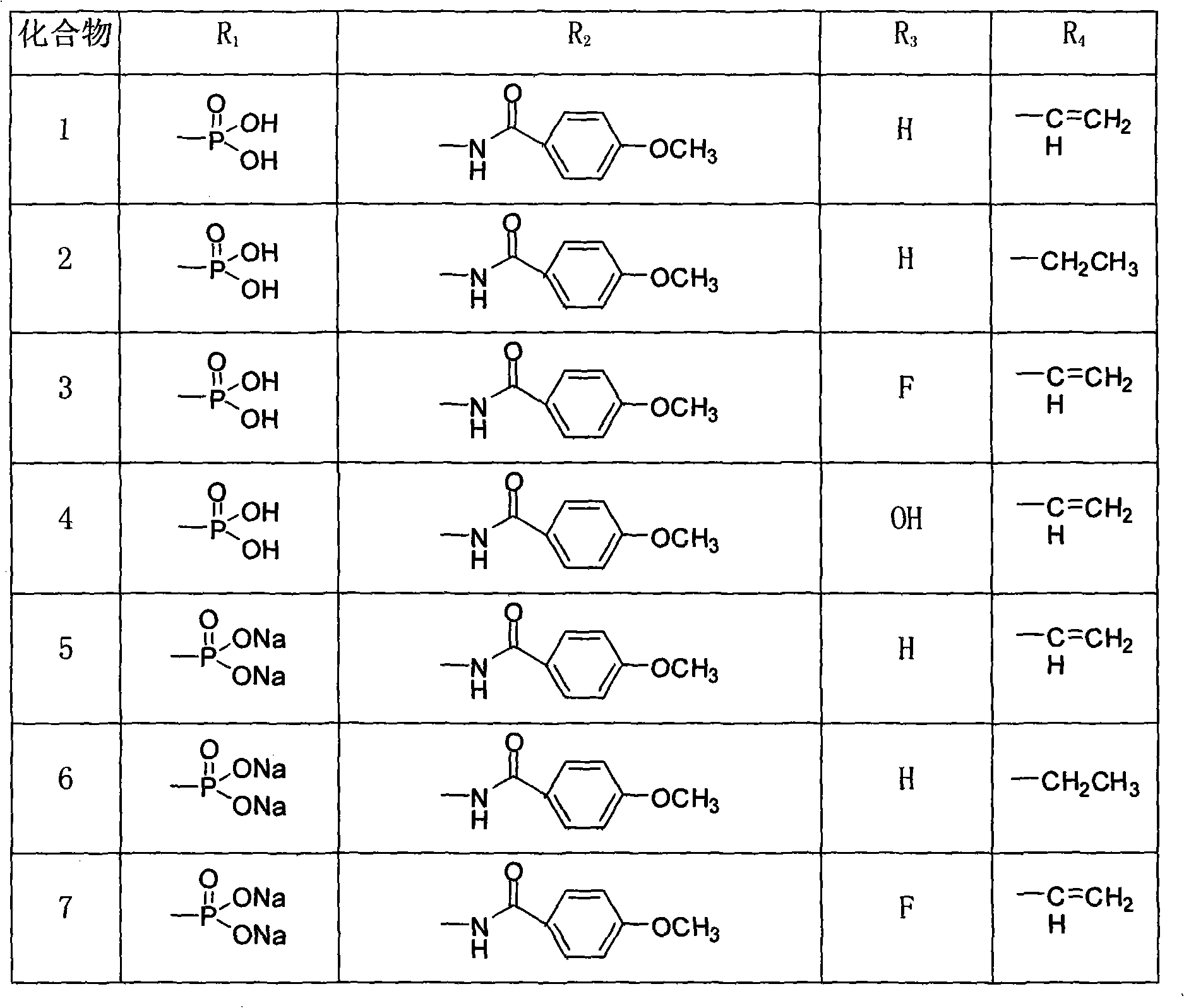
Pleuromutilin phosphate compounds, medicinal composition thereof, preparation method thereof and application thereof
A technology of pleuromutilin and phosphate esters, applied in the fields of pharmacology, drug synthesis and pharmacology, can solve the problems of first-pass metabolism and poor water solubility, and achieve the effect of reducing adverse effects and improving water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Example 1: 14-[N-(4-methoxybenzoyl)]-carbamoylmutriptyline-11-dihydrogen phosphate (compound 1)
[0082] (a) Preparation of 14-[N-(4-methoxybenzoyl)]-carbamoylmutriptyline-11-dibenzyl phosphate
[0083]
[0084] Under nitrogen protection, 14-[N-(4-methoxybenzoyl)]-carbamylmutriptyline (200mg, 0.4mmol) and 1-hydrotetrazolium (140mg, 2.0mmol) were dissolved in In 10 mL of dry dichloromethane, stir at room temperature, add dibenzyl N, N'-diisopropyl phosphoramidite (695 mg, 2.0 mmol), continue the reaction for 2 hours, cool to -78 ° C, and add to the reaction A dry dichloromethane solution (5 mL) of m-chloroperoxybenzoic acid (500 mg, 2.0 mmol) was added dropwise to the solution, stirred for 30 minutes, then heated to -20°C and stirred for 1 hour. Slowly add 10% sodium bisulfite aqueous solution (20mL) dropwise to the reaction solution to quench the reaction, add dichloromethane (20mL), separate the layers, and use sodium bisulfite solution, saturated sodium carbonate ...
Embodiment 2
[0088] Example 2 14-[N-(4-methoxybenzoyl)]-carbamoylmutriptyline-11-disodium phosphate (compound 5)
[0089]
[0090] Example 1(b) compound (2g, 3.47mmol) and sodium acetate (0.57g, 6.93mmol) were stirred in water (20mL) until clarified, filtered, and the filtrate was lyophilized to obtain a white solid, which was recrystallized from ethanol and vacuum Drying gave the title compound (1.27 g, 59%). 1 H-NMR (D 2 O, ppm): δ7.96 (d, 2H), 7.15 (d, 2H), 6.43 (dd, 1H), 5.79 (d, 1H), 5.33 (dd, 2H), 4.41 (m, 1H), 3.95 (s,3H), 2.45(q,1H), 0.98-2.6(m,12H), 1.61(s,3H), 1.29(s,3H), 1.14(d,3H), 0.84(d,3H).
Embodiment 314
[0091] Example 314-[N-(4-methoxybenzoyl)]-carbamoyl-19,20-dihydromutriptyline-11-dihydrogen phosphate (compound 2)
[0092]
[0093] Example 1 (a) compound 14-[N-(4-methoxybenzoyl)]-carbamoyl-mutriptyline-11-dibenzyl phosphate (250mg, 0.33mmol) was dissolved in anhydrous methanol (20mL), add 35mg10%Pd / C, shake table catalytic reduction under 3atm hydrogen pressure for 5 hours. 20 / 45μm, the mobile phase was methanol: 0.25% trifluoroacetic acid aqueous solution = 20:80-50:50 gradient elution), and the title product (84.9 mg, 44.5%) was obtained as a white solid powder after lyophilization of the residual aqueous phase. 1 H-NMR (MeOD, ppm): δ7.86 (d, 2H), 7.01 (d, 2H), 5.72 (d, 1H), 4.31 (m, 1H), 3.83 (s, 3H), 2.54 (q, 1H), 0.88-2.4(m, 17H), 1.49(s, 3H), 1.12(s, 3H), 0.86(d, 3H), 0.75(d, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com