Method for preparing epoxy cyclohexane through catalytic epoxidation of cyclohexene
A cyclohexene catalytic ring, epoxy cyclohexane technology, applied in organic chemistry and other directions, can solve the problems of low selectivity, low atom economy, and high raw material consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
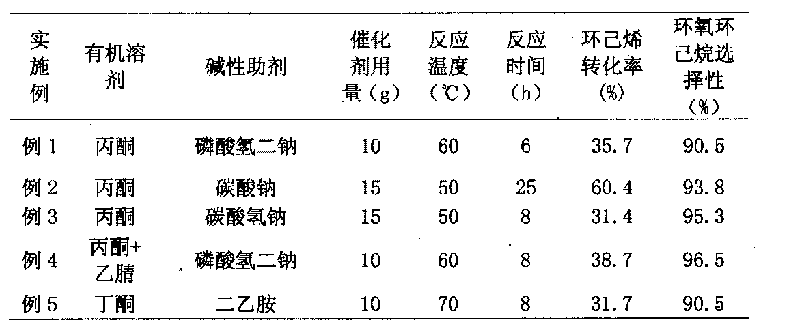
[0023] Mix 800 milliliters of acetone and 1 mole of cyclohexene with 10 grams of titanium-silicon molecular sieve in a reaction flask, add 2 grams of disodium hydrogen phosphate, raise the temperature to 60°C, and add 1 mole of hydrogen peroxide dropwise in 2 hours. 6 hours.
Embodiment 2
[0025] Mix 1200 milliliters of acetone and 1.5 moles of cyclohexene with 15 grams of titanium-silicon molecular sieve in a reaction flask, add 2 grams of sodium carbonate, raise the temperature to 50°C, and add 1.5 moles of hydrogen peroxide dropwise in 2 hours, and react for 25 hours .
Embodiment 3
[0027] Mix 1200 milliliters of acetone and 3.0 moles of cyclohexene with 15 grams of titanium-silicon molecular sieves in a reaction flask, add 3 grams of sodium bicarbonate, raise the temperature to 50°C, and add 1.5 moles of hydrogen peroxide dropwise in 2 hours. Reaction 8 Hour.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com